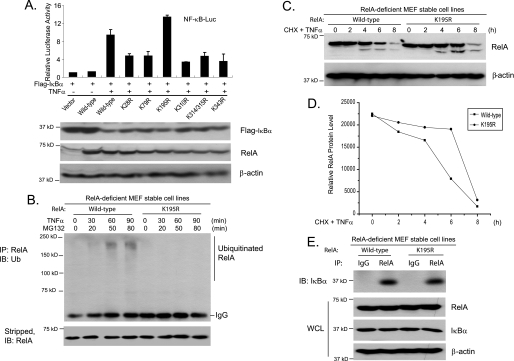
FIGURE 2.
RelA Lys-195 mediates TNFα-induced RelA polyubiquitination. A, effects of overexpression of RelA lysine-to-arginine mutants on TNFα-induced NF-κB activation. Expression vectors encoding IκBα, NF-κB luciferase (NF-κB-Luc) reporter, and Renilla luciferase plasmids were cotransfected into RelA-deficient MEF cells along with wild-type RelA and lysine-to-arginine mutants, and then the cells were either untreated or treated with TNFα (1 ng/ml) for 6 h. The relative luciferase activity was measured and normalized with the Renilla activity. Error bars indicate standard deviations from triplicate experiments. B, TNFα induces RelA polyubiquitination at Lys-195. RelA-deficient MEF cells with stable expression of wild-type RelA or the K195R mutant were either untreated or treated with TNFα (10 ng/ml) for the times indicated in the presence of MG132 and subsequently lysed. RelA proteins in the cell lysates were immunoprecipitated (IP) with anti-RelA antibodies and immunoblotted (IB) with anti-Ub antibodies to detect the presence of ubiquitinated RelA. C, RelA Lys-195 is required for TNFα-induced RelA degradation. RelA-deficient MEF cells with stable expression of wild-type RelA or the K195R mutant were either untreated or treated with TNFα (10 ng/ml) for the time points indicated in the presence of cycloheximide (CHX) and subsequently lysed. The whole cell lysates (WCL) were immunoblotted with anti-RelA antibodies to detect the stability of RelA. D, quantification of results from C. E, Wild-type RelA and K195R have comparable binding affinities for IκBα. Wild-type RelA and K195R proteins in the reconstituted MEF cells were immunoprecipitated with anti-RelA antibodies and immunoblotted with anti-IκBα antibodies to detect the presence of IκBα in the immunoprecipitates.