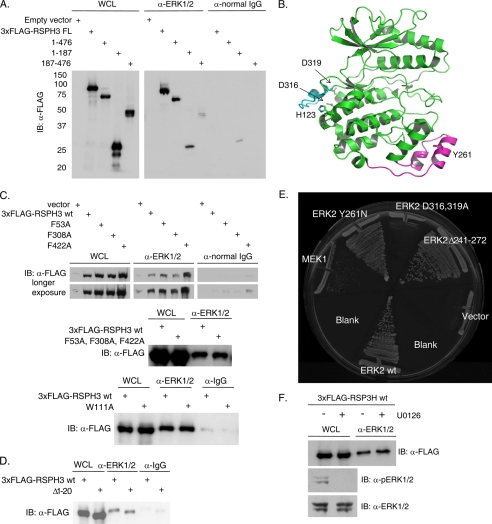
FIGURE 2.
ERK CD region is involved in the RSPH3 interaction. A, endogenous ERK1/2 was immunoprecipitated from HEK293 cells expressing 3xFLAG-tagged RSPH3 truncation mutants, residues 1–476, 1–187, and 187–476. Immunoprecipitation with normal rabbit IgG was used as a negative control. Precipitates were immunoblotted (IB) with anti-FLAG antibody. The whole cell lysate (WCL) lane contains 5% of the input for the immunoprecipitations. B, ribbon representation of ERK2 (green) bound via its CD domain to a peptide derived from the hematopoietic tyrosine phosphatase HePTP (cyan) (Protein Data Bank code 2gph). The MAPK insert is highlighted in magenta, with Tyr-261 indicated. C, three putative FXF motifs within 3xFLAG-RSPH3 were mutated singly (top panel) or in combination (middle panel), and a WXY motif within 3xFLAG-RSPH3 was mutated (bottom), and all were tested for ERK1/2 binding. Endogenous ERK1/2 immunoprecipitates were immunoblotted with anti-FLAG. Immunoprecipitation from cells transfected with empty vector or with normal rabbit IgG were used as negative controls. The whole cell lysate lane contains 5% of the input for immunoprecipitation. D, experiment as in C with a truncation of the first 20 residues of 3xFLAG-RSPH3. E, pairwise yeast two-hybrid analysis of mouse RSP3 interaction with wild-type (wt) ERK2 or ERK2 mutants. Yeast containing RSP3 were co-transformed with wild-type MEK1 or empty vector as controls. F, HEK293 cells expressing 3xFLAG-RSPH3 were treated with 10 μm U0126 or diluent for 10 min prior to lysis. Endogenous ERK1/2 were immunoprecipitated, and the precipitates were immunoblotted (IB) with anti-FLAG. The whole cell lysate lane contains 5% of the input. Phosphorylated and total ERK1/2 were immunoblotted in the lysates. n = 3. Experiments in each panel were performed three times, except the two-hybrid test which was performed twice.