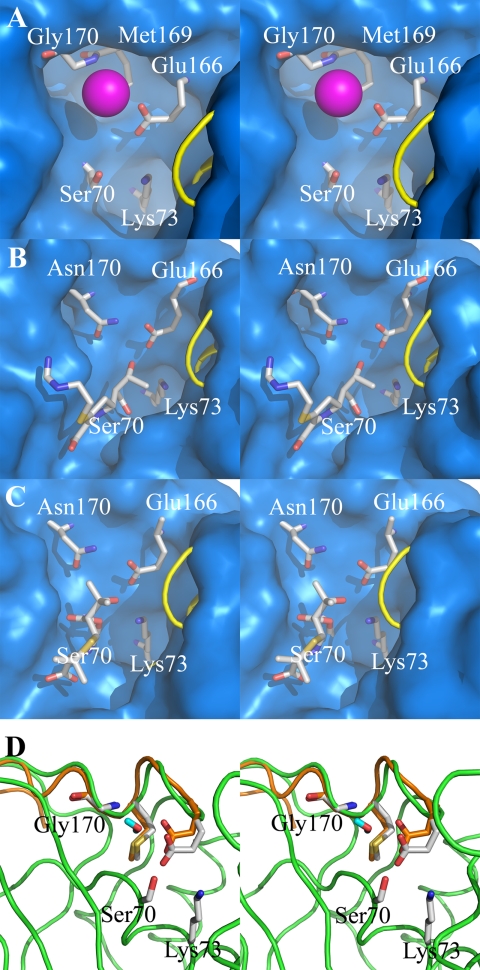
FIGURE 2.
Crystal and molecular dynamics structures of class A carbapenemases. A stereoview representation of the active-site conformations for the crystal structure of the GES-1 β-lactamase (A), the acyl-enzyme species of imipenem bound to the class A TEM-1 β-lactamase from E. coli (B) (30), 6α-(hydroxypropyl)penicillanate bound to the NMC-A β-lactamase from E. cloacae (C) (25), and molecular motions of two extreme conformations of GES-1 from dynamics simulations (D). The active sites (A–C) are depicted as Connolly solvent-accessible surfaces, with residues at positions 70, 73, 166, 169, and 170 shown as capped sticks. (Carbon atoms are colored in gray, nitrogen in blue, oxygen in red, and hydrogen in cyan, with a loop shown as a yellow tube for visual access to the active site.) A conserved water molecule is shown between residues 170 and 166 (A) as a sphere (in magenta). The role of the 6α-(hydroxypropyl) moiety in the structure of C is the same as that of the 6α-(hydroxyethyl) group of imipenem shown in B; namely, an impediment to the travel of the hydrolytic water molecule to the acyl-enzyme carbonyl. The deacylation step is rate-limiting in these two examples. Two extreme conformations are shown in D with motion of residue 166 Cα by ∼1 Å. The protein and Ω-loop of one conformer are colored in green and the Ω-loop of another conformer in orange, with residues shown in capped sticks. (Atoms are colored the same as in A–C, except the carbon atoms from the second conformer that are colored in orange.) The water molecule shown in capped stick is from the second conformer.