Abstract
Lysosomal storage diseases (LSD) are metabolic disorders characterized by accumulation of undegraded material. The mucopolysaccharidoses (MPS) are LSDs defined by the storage of glycosaminoglycans. Previously, we hypothesized that cells affected with LSD have increased energy expenditure for biosynthesis because of deficiencies of raw materials sequestered within the lysosome. Thus, LSDs can be characterized as diseases of deficiency as well as overabundance (lysosomal storage). In this study, metabolite analysis identified deficiencies in simple sugars, nucleotides, and lipids in the livers of MPSI mice. In contrast, most amino acids, amino acid derivatives, dipeptides, and urea were elevated. These data suggest that protein catabolism, perhaps because of increased autophagy, is at least partially fulfilling intermediary metabolism. Thus, maintaining glycosaminoglycan synthesis in the absence of recycled precursors results in major shifts in the energy utilization of the cells. A high fat diet increased simple sugars and some fats and lowered the apparent protein catabolism. Interestingly, autophagy, which is increased in several LSDs, is responsive to dietary intervention and is reduced in MPSVII and MPSI mice fed a high fat diet. Although long term dietary treatment improved body weight in MPSVII mice, it failed to improve life span or retinal function. In addition, the ventricular hypertrophy and proximal aorta dilation observed in MPSVII mice were unchanged by a high fat, simple sugar diet. As the mechanism of this energy imbalance is better understood, a more targeted nutrient approach may yet prove beneficial as an adjunct therapy to traditional approaches.
Lysosomal storage disease (LSD)2 typically results from a genetic deficiency of an acid hydrolase (1). The material usually degraded by the enzyme now accumulates in the lysosomes of cells throughout the body. In normal cells, some proportion of the degraded material is exported to the cytosol for reuse, thereby reducing the energy burden on the cell (2, 3). In the case of LSDs, more energy must be diverted to the synthesis of raw material because of the impaired recycling. Thus, this class of disorders presents with an energy imbalance caused by a simultaneous excess of stored material and a deficiency of raw material (4).
Deficiencies in lysosomal enzymes involved in glycosaminoglycan (GAG) catabolism result in the mucopolysaccharidoses (MPS) (5). The biochemical, histological, and clinical phenotypes of MPS are likely due to a combination of the adaptations to both lysosomal storage and a deficiency of recycled monosaccharides. Maintaining a normal rate of GAG biosynthesis would require newly imported or synthesized monosaccharides, irrespective of the adaptations to stored material. The increased demand for GAG precursors is likely to be considerable. It has been shown previously in cultured cells that reutilization of catabolites from lysosomal GAG degradation is substantial (2). Therefore, the increased energy burden required for de novo synthesis of GAG precursors might be expected to reduce the levels of stored energy throughout the body. Consistent with this hypothesis, we and others have shown that a number of lysosomal storage diseases have significantly reduced adiposity (4, 6–8). We showed that the reduced adiposity was not the result of reduced food intake, higher metabolic rate, or lipid malabsorption (4). Furthermore, the missing lipid energy was not found in other tissues (4). However, the total caloric content in the liver was normal and in the form of carbohydrates other than glycogen (4). Enzyme replacement therapy reduced the levels of carbohydrates suggesting that their identity was glycosaminoglycans (4). These data strongly argued that energy was being diverted from potential storage as fat in adipocytes to storage in lysosomes as undegraded glycosaminoglycans. It is unclear how diseased cells are adapting to the energy diversion resulting from the block in recycling and what effect this has on the whole organism.
In this study, we identified a number of biochemical and metabolic abnormalities associated with two MPS disorders. The data are consistent with the idea that physiologic malnutrition (9) and altered energy flow (4) are common adaptations in many, if not most LSDs. We also determined the effects of nutritional supplementation on these changes. Many of the metabolic abnormalities approached normal when the animals were placed on a high fat, simple sugar diet. Of particular interest is the fact that autophagy was responsive to dietary intervention and approached normal levels when the animals were fed a high fat diet. This identifies at least one mechanism that contributes to the increased autophagy observed in a number of diverse LSDs (10–19). Although long term dietary intervention in MPSVII mice resulted in only minor clinical improvements, a more thorough understanding of this defect may ultimately lead to more targeted metabolic interventions that provide clinical benefit as an adjunct therapy.
EXPERIMENTAL PROCEDURES
Animal Procedures and Diets
All animal experiments were approved by the Institutional Animal Care and Use Committee at Washington University. The MPSI and MPSVII colonies are maintained as pedigreed colonies through strict brother-sister matings at Washington University by M. S. S. The genotypes of MPSI, MPSVII, and normal controls were identified by PCR. Depending on the experiment, mice were fed either a “22% fat” (PicoLab Mouse Chow 20 number 5058, LabDiet/PMI Nutritional International, St. Louis, MO), “13% fat” (PicoLab Mouse Chow 20 number 5053), “42% fat” (number TD88137, Harlan Teklad, Madison, WI), or “Modified 42% fat” (number TD07203, Harlan Teklad) diet. The Modified 42% fat diet is essentially the same as the 42% fat diet with the addition of 1% soybean oil in exchange for an equivalent amount of milk fat. The Modified 42% fat diet was used for long term feeding. For experiments requiring fasting, animals had food withheld for 4 h. To examine the role of diet in longevity, MPSVII and control animals were weaned onto either the 13% fat diet or the Modified 42% fat diet. Animals were weighed weekly starting at weaning until death or sacrificed when moribund.
Metabolite Analysis
Five-month-old MPSI and control mice reared on the 22% fat diet were fasted for 4 h. Liver tissue was harvested, flash-frozen in liquid nitrogen, and then stored at −80 °C. Over 1,500 metabolites were quantified using gas chromatography/mass spectrometry and liquid chromatography/mass spectrometry in combination with proprietary software (Metabolon Inc., Durham, NC). Separate cohorts of MPSI and control mice were reared for 4.5 months on the 22% fat diet. They were then given the Modified 42% fat diet for 4 weeks and sacrificed after a 4-h fast.
Western Blotting
Liver samples from 5-month-old MPSI and control animals that were fed a 42% fat diet for 2 weeks or maintained continuously on the 22% fat diet were harvested, flash-frozen in liquid N2, and then stored at −80 °C. Liver samples from 3½-month-old MPSVII and control mice fed either a Modified 42% fat diet or 13% fat diet were harvested, flash-frozen in liquid N2, and stored at −80 °C. Liver samples were homogenized in ice-cold RIPA buffer (0.05 m Tris (pH 7.4), 0.15 m NaCl, 0.25% sodium deoxycholate, 1% Nonidet P-40; 3 μl per mg tissue) in the presence of proteinase inhibitors (P8340, Sigma) at 1:60 dilution. Debris was pelleted (16,000 × g) twice in a microcentrifuge at 4 °C. The supernatant was aliquoted and frozen at −80 °C. Protein concentration was determined by the Coomassie dye binding assay (Bio-Rad). 25 μg of total protein was boiled in Laemmli buffer with 5% β-mercaptoethanol and loaded onto a NuPAGE 12% BisTris gel (Invitrogen). The gel was run and transferred essentially as described previously (4). Membranes were incubated with an anti-LC3 rabbit polyclonal primary antibody (NB100-2220, 2 μg/ml, Novus Biologicals, Littleton, CO) in TBST with 10% milk (w/v) overnight at 4 °C. Membranes were washed three times for 15 min each in TBST. The secondary horseradish peroxidase-conjugated donkey anti-rabbit antibody (GE Healthcare) was diluted 1:5000 in TBST and incubated for 45 min. Membranes were washed three times for 5 min each in TBST and incubated with ECL Plus Western blotting detection system (GE Healthcare). Images were captured using a variety of exposure times on Biomax MR-1 film (Eastman Kodak Co.). Membranes were stripped using stripping buffer at 55 °C for 30 min. After stripping, membranes were washed three times for 5 min each with TBST and blocked for 1.5 h at room temperature in TBST, 10% milk (w/v). An anti-β-actin antibody (A5441, 1:2000, Sigma) was incubated with the membranes for 1 h at room temperature. After three washes for 15 min, membranes were incubated with a secondary horseradish peroxidase-conjugated sheep anti-mouse antibody (NA931V, 1:200, GE Healthcare) for 45 min. Images were developed and captured as described above. Finally, the gels were stained with Ponceau S and scanned to ensure consistent loading across lanes. Bands corresponding to LC3I and LC3II based on size could be competed away by co-incubating 20 μg of anti-LC3 antibody with 20 μg of LC3-specific blocking peptide prior to Western blottings (NB100-2220PEP, Novus Biologicals).
Echocardiography
MPSVII and control mice were weaned onto the 13% fat diet or Modified 42% fat diet. At 13 weeks of age, mice were analyzed by echocardiography. Cardiac ultrasound was performed using the Vevo770 Ultrasound System (VisualSonics, Inc, Toronto, Ontario, Canada) essentially as described previously (20). Briefly, anesthetized animals (continuous gaseous 1% isoflurane) were imaged in the supine position, and heart rate, body temperature, and respiratory rate were monitored. A variety of transducers with different ultrasound frequencies (30–60 MHz) were used to monitor different regions of interest. From each mouse we obtained two-dimensional, M-mode, and Doppler ultrasound from different orientations. When appropriate, data were normalized to body weight.
Electroretinography
At 14 weeks of age MPSVII and control mice reared on either the 13% fat diet or Modified 42% fat diet were analyzed by flash electroretinography. Retinal function was examined using the Tucker-Davis System 3 Complete ABR/OAE workstation (Tucker-Davis Technologies, Alachua, FL) essentially as described previously (21). Briefly, mice were dark-adapted overnight prior to rod/cone analysis. Mice were then exposed to light flashes (10 μs duration at 76.2 candelas·s/m2 intensity). The average response to five flashes of light was obtained at 0.1 Hz. For cone analysis, mice were then light-adapted for 10 min using a light intensity of 72.7 candelas/m2. The average response to 50 flashes of light was obtained at 1 Hz. The b-wave amplitude (in microvolts) was averaged separately for dark- and light-adapted conditions by subject group.
Statistical Analysis
Metabolomics data were analyzed by two-way ANOVA. Three different comparisons (diet main effect, genotype main effect, and diet by genotype interaction) were made. Longevity data were analyzed using Kaplan-Meier analysis. Body weight data were analyzed separately for control and MPSVII mice using mixed model ANOVA (SAS 9.2). The outcome was weight, and the predictors were diet, time (as linear and quadratic terms), and diet by time (both linear and quadratic) interaction. All other data were analyzed using two-tailed Student's t test assuming equal variance. p values <0.05 were considered statistically significant for all analyses.
RESULTS
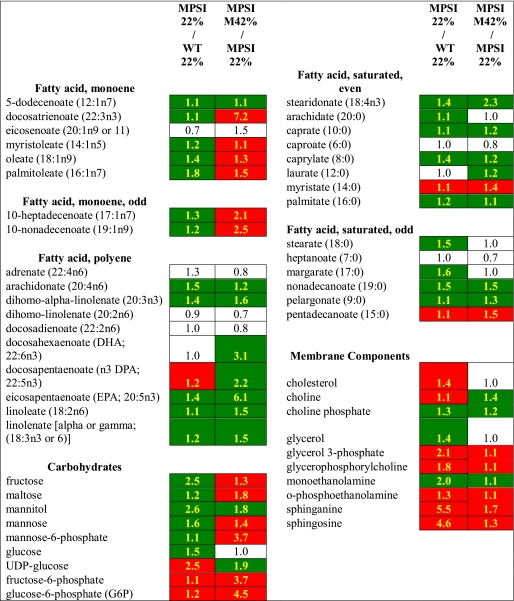
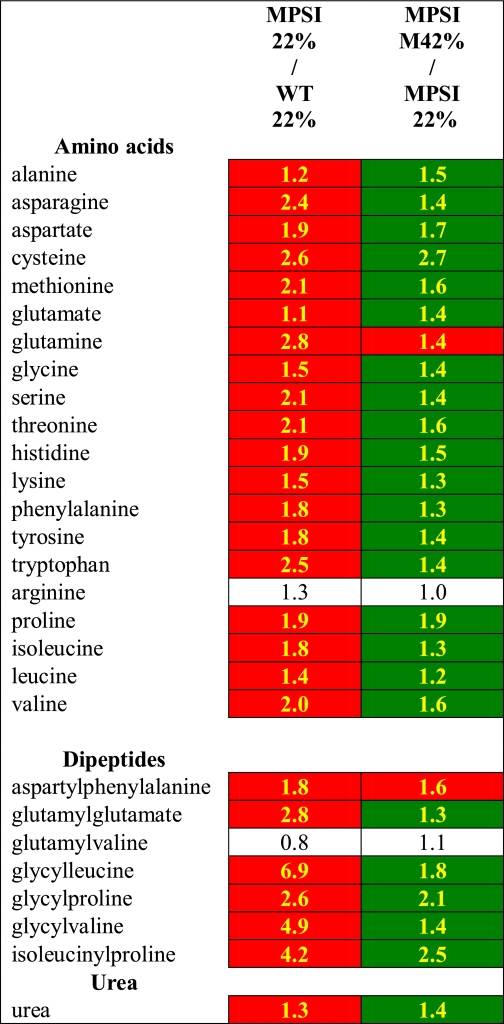
Liver metabolites from fasting MPSI and normal control animals maintained on the 22% fat diet were identified and quantified by metabolomic analysis. There were significant differences detected in 224 metabolites between the two groups (supplemental Table 1). Simple sugars such as fructose, glucose, mannose, mannose 6-phosphate, and ribose were all reduced (p < 0.05) in comparison with controls (Table 1). The biosynthetic molecule UDP-glucose was increased as well as both fructose 6-phosphate and glucose 6-phosphate. Nucleotide metabolites were uniformly decreased with the exception of UMP, UDP, AMP, and adenine which were significantly increased. Lipid metabolites were broadly decreased with some exceptions, particularly the membrane components cholesterol, sphinganine, sphingosine, and glycerolipids. Most amino acids, a number of dipeptides, many amino acid derivatives, and urea were increased in MPSI livers suggesting an increase in protein catabolism (Table 2). Thus, compared with normal control animals, MPSI mice have deficiencies of carbohydrates and lipids and an apparent increase in protein degradation.
TABLE 1.
Livers from MPSI mice are deficient in carbohydrates and lipids and respond to an energy-rich diet
All values reported are fold changes. Data highlighted in green are significantly reduced, and data highlighted in red are significantly increased (p < 0.05). Values in white boxes indicate no significant differences between the two groups. 22% indicates 22% fat diet. M42% indicates modified 42% fat diet.
TABLE 2.
Livers from MPSI mice have increased levels of amino acids, dipeptides, and urea and respond to an energy-rich diet
All values reported are fold changes. Data highlighted in green are significantly reduced, and data highlighted in red are significantly increased (p < 0.05). Values in white boxes indicate no significant differences between the two groups. 22% indicates 22% fat diet. M42% indicates modified 42% fat diet.
We hypothesized that the apparent increase in protein degradation was to maintain intermediary metabolism in response to the carbohydrate and lipid deficiencies. To test this hypothesis, 4.5-month-old MPSI and normal control mice were fed the Modified 42% fat diet for 1 month. Liver metabolite analysis detected significantly increased levels of the simple sugars fructose, maltose, mannose, and mannose 6-phosphate in MPSI mice fed the Modified 42% fat diet compared with MPSI controls fed the 22% fat diet (Table 1). In contrast, glucose was unchanged, and ribose was further reduced. Both fructose 6-phosphate and glucose 6-phosphate were significantly elevated in Modified 42% fat diet MPSI mice compared with MPSI animals reared solely on the 22% fat diet. In general, nucleotide metabolite levels were further reduced; however, inosine, adenosine, guanosine, GMP, cytidine, and UMP were all elevated. The membrane components sphinganine, sphingosine, and glycerolipids were significantly increased, whereas cholesterol levels remained unchanged. Lipids were further reduced with the exception of monoenes that were elevated. Most amino acids, the detected dipeptides, many of the amino acid derivatives, and urea were all decreased in MPSI mice fed the Modified 42% fat diet compared with mice fed only the 22% fat diet (Table 2).
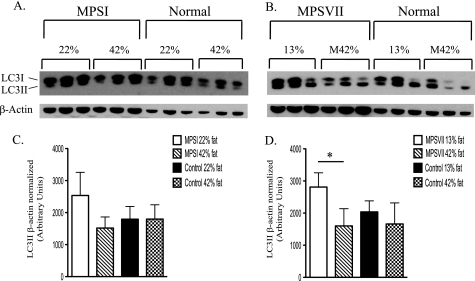
Increased autophagy has been observed in a number of LSDs (10–19). The apparent increase in protein catabolism observed in this study could be mediated, in part, by an increase in autophagy. Therefore, we tested whether the 42% fat diet, which appeared to reduce protein catabolism in MPSI mice, would decrease the level of LC3II, a molecular marker of autophagy. Two groups of MPSI mice were fed the standard 22% fat diet from weaning until 4.5 months of age. At that time, one group was fed the 42% fat diet for 2 weeks. Western blotting of nonfasted liver homogenates revealed a trend (p = 0.080) toward reduced levels of LC3II in the 42% fat diet-treated group (Fig. 1A). MPSVII mice had a significant decrease (p < 0.039) in LC3II after eating the Modified 42% fat diet from weaning until 14 weeks of age when compared with MPSVII mice fed the 13% fat diet (Fig. 1B). Thus, LC3II levels in both MPSI (p = 0.08) and MPSVII (p < 0.04) were reduced in response to the increased energy intake. The decrease in the apparent protein catabolism observed in MPSI mice on the Modified 42% fat diet is consistent with the decrease in autophagy. There was no significant difference in the levels of LC3II between the normal control animals on the higher fat diet and normal control animals on the lower fat diet in either experiment.
FIGURE 1.
Autophagy in MPSI and MPSVII liver is reduced by a high fat, simple sugar diet. MPSI (A) and MPSVII (B) LC3I and LC3II levels in liver were determined by Western blotting. Mice were fed the 22% fat diet (22%, A), the 42% fat diet (42%, A), the 13% fat diet (13%, B), or the Modified 42% fat diet (M42%, B). The dietary-induced change in LC3II levels in the MPSI (C) and MPSVII (D) Western were determined by densitometry and normalized to β-actin. Data shown are the average of 3 separate mice/diet ± 1 S.D. *, p < 0.05.
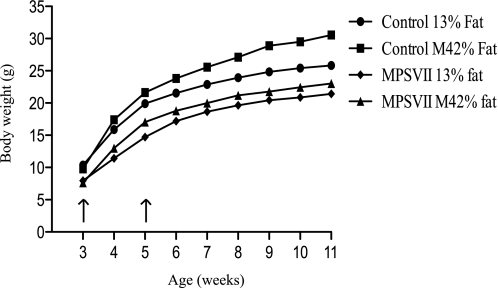
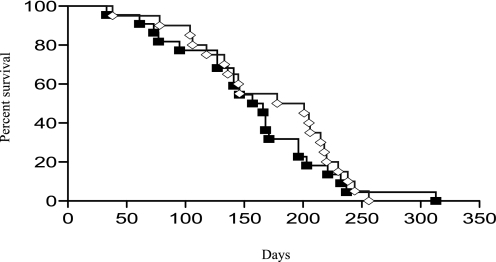
The Western Diet improved several metabolic parameters in MPSI and MPSVII mice. To determine the clinical impact of a high fat, simple sugar diet, MPSVII and normal control mice were weaned onto either the Modified 42% fat diet or the 13% fat diet and fed the diet for life. MPSVII mice fed the 42% fat diet weighed significantly more than 13% fat diet-treated MPSVII mice (Fig. 2). Interestingly, the rate of weight gain in the Modified 42% fat diet-treated MPSVII mice was significantly greater (p < 0.002) between 3 and 5 weeks of age when compared with MPSVII mice maintained on the 13% fat diet. Although the body weights of the MPSVII mice on the Modified 42% fat diet remained significantly greater for the 11-week period, there was no significant difference in the rate of weight gain between the two groups beyond 5 weeks of age. In contrast, the rate of weight gain in Modified 42% fat diet-treated normal controls was significantly greater throughout the entire 11-week experiment when compared with normal controls maintained on the 13% fat diet. Although the MPSVII mice on the Modified 42% fat diet had significantly increased body weight during the entire 11-week study period, this had no significant impact on life span (Fig. 3). The median life span of MPSVII mice fed the 13% fat diet was 189.5 days and the Modified 42% fat diet was 161.5 days. There was no significant effect of diet on the life span of the normal control animals for the duration of this study (10 months).
FIGURE 2.
Increased MPSVII body weight with prolonged high fat, simple sugar feeding. MPSVII and normal control mice were weaned onto either the 13% fat diet (13% fat) or the Modified 42% fat diet (M42% fat)and weighed weekly. MPSVII 13% fat (n = 18–20, diamonds), MPSVII M42% fat (n = 19–22, triangles), normal control 13% fat (n = 22–23, circles), and normal control M42% fat (n = 22, squares) are as shown. Data shown are the average at each time point. Both normal control and MPSVII body weight on the Modified 42% fat diet were greater (p < 0.05) than when fed the 13% fat diet. Arrows indicate the interval where the rate of weight gain in MPSVII mice fed the Modified 42% fat diet (p < 0.05) was greater than MPSVII mice fed the 13% fat diet.
FIGURE 3.
MPSVII longevity does not improve with dietary intervention. MPSVII longevity on the Modified 42% fat diet (n = 22, M42% fat diet, gray line, open diamonds) was compared with MPSVII fed the 13% fat diet (n = 20, 13% fat diet, black line, filled squares).
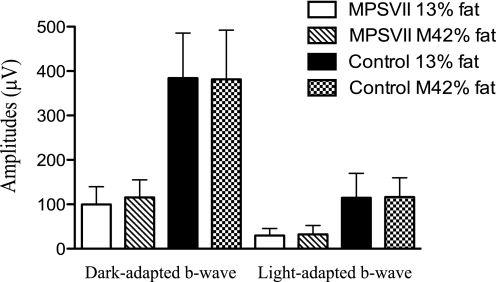
We documented the effects of the Modified 42% fat diet on cardiac physiology and retinal function in MPSVII mice. We chose to study the cardiac physiology because the heart primarily uses lipid energy for fuel (22, 23). Furthermore, carnitine deficiency, a lipid metabolism deficiency, results in cardiac hypertrophy (24). Significant cardiac hypertrophy is seen in MPSVII mice (25). As determined by echocardiography, MPSVII mice have left ventricular hypertrophy as indicated by the increase in left ventricular mass, increased wall thickness, and increased left ventricular lumen. None of these abnormal parameters were altered by dietary intervention (Table 3). MPSVII mice also have significant dilation of the proximal aorta (26), which was not influenced by diet (Table 3). We chose retinal function for study because of the high energy demands of the tissue and limited glucose stores (26). MPSVII mice have impaired retinal function as measured by electroretinography (27) and suffer hypoglycemia (4). However, we found no significant improvement in electroretinographic response in dietary-treated MPSVII mice (Fig. 4). In summary, whereas a high fat, simple sugar diet improved a number of biochemical and metabolic parameters as well as body weight in MPSVII mice, it had little clinical effect.
TABLE 3.
MPSVII have a hypertrophic heart that was resistant to treatment
All values reported are averages ± 1 S.D. 13% is the 13% fat diet, and M42% is the Modified 42% fat diet. The abbreviations used are as follows for LV measurements: LVPW, left ventricle posterior wall thickness; LVIDd, left ventricular internal diameter in diastole; LVM, left ventricular mass; FS, fractional shortening. The abbreviations used are as follows for Doppler: Tei index, cardiac performance index. The abbreviations used are as follows for aortic diameters: AV, aortic valve; SV, sinus of Valsalva; STJ, sinotubular junction; AscAo, ascending aorta; Arch, aortic arch; DescAo, descending aorta. The abbreviations used are as follows for aortic regurgitation: AI DescAo Retrogr, retrograde diastolic flow profile in the descending aorta.
| Control, 13%(n = 8–11) | MPSVII, 13%(n = 9–12) | MPSVII, M42%(n = 9–10) | |
|---|---|---|---|
| Body weight (g) | 26.8 ± 2.3 | 20.9 ± 1.8a | 22.7 ± 2.4b |
| Heart rate (beats/min) | 478 ± 42 | 518 ± 65 | 560 ± 56b |
| LV measurement | |||
| LVPWdc | 0.027 ± 0.003 | 0.039 ± 0.004a | 0.037 ± 0.006b |
| LVIDdc | 0.148 ± 0.010 | 0.180 ± 0.020a | 0.165 ± 0.017b |
| LVMc | 3.7 ± 0.7 | 5.1 ± 0.8a | 4.9 ± 0.9b |
| FS | 31.9 ± 3.0 | 35.6 ± 7.2 | 36.8 ± 6.9b |
| Doppler | |||
| Tei index | 0.60 ± 0.07 | 0.53 ± 0.05a | 0.55 ± 0.09 |
| Aortic diameters | |||
| AV (mm/g)c | 0.048 ± 0.005 | 0.063 ± 0.005a | 0.060 ± 0.006b |
| SV (mm/g)c | 0.067 ± 0.007 | 0.103 ± 0.012a | 0.094 ± 0.013b |
| STJ (mm/g)c | 0.049 ± 0.005 | 0.080 ± 0.011a | 0.072 ± 0.008b |
| AscAo (mm/g)c | 0.049 ± 0.004 | 0.098 ± 0.011a | 0.106 ± 0.019b |
| Arch (mm/g)c | 0.048 ± 0.003 | 0.075 ± 0.005a | 0.078 ± 0.012b |
| DescAo (mm/g)c | 0.041 ± 0.004 | 0.067 ± 0.007a | 0.062 ± 0.006b |
| Aortic regurgitation | |||
| AI DescAo retrograde | 47.46 ± 12.30 | 96.44 ± 60.56a | 119.25 ± 63.05b |
Data from MPSVII mice on 13% diet are significantly (p < 0.05) different from control mice on a 13% diet.
Data from MPSVII mice on M42% diet are significantly (p < 0.05) different from control mice on 13% diet.
Indexed to body weight.
FIGURE 4.
MPSVII retinal function was not improved with the modified 42% fat diet. Dark- and light-adapted b-wave amplitudes (μV) were determined in 3.5-month-old MPSVII and normal control mice fed the 13% fat diet (13% fat) or the Modified 42% fat diet (M42% fat). MPSVII 13% fat (n = 6–7, open bars), MPSVII M42% fat (n = 8–9, hatched bars), control 13% fat (n = 11, filled bars), and control M42% fat (n = 12, checkered bars) are shown. Data shown are the average ± 1 S.D.
DISCUSSION
Lysosomal storage disorders are typically defined as diseases of overabundance, i.e. lysosomal accumulation. It is almost certain that lysosomal storage plays a role in disease progression. However, an important function of the lysosome is to recycle macromolecules into their constituent compounds for reuse. A deficiency of a lysosomal enzyme leads to an accumulation of intact or only partially degraded substrates but simultaneously leads to a deficiency of the building blocks of those substrates due to interrupted recycling. Recycling macromolecules through lysosomal degradation is an energy-efficient means of generating raw materials rather than using energy-intensive synthetic mechanisms, thus saving the cell energy and resources. Therefore, to maintain homeostasis a cell with an LSD would be expected to import more raw materials and utilize more energy for the de novo synthesis of new macromolecule precursors. The increased use of energy and raw material to maintain normal function would be expected to reduce the amount of energy that could be stored for future use. In fact, we showed previously that an energy imbalance in the form of decreased adiposity is a common feature in a number of LSDs (4). Decreased adiposity has also been reported in the mouse models of Sly syndrome, Wohlman disease, and mucolipidosis IV (7–9). It has also been shown that the CUP-5 mutant strain of Caenorhabditis elegans has a profound energy imbalance (13). Interestingly, CUP-5 is the C. elegans orthologue of the mammalian mucolipin IV, which leads to mucolipidosis IV. How this energy imbalance affects disease progression is unknown. A more thorough knowledge of how cells affected by an LSD are adapting to both the accumulated material and the deficiency of recycled metabolites will likely be important to understanding how the disease phenotypes arise.
In this study, we used an unbiased metabolomics approach to identify molecules that might be altered in the livers of MPSI mice. Liver was chosen for study because of the severity of disease in the organ, the limited number of cell types, and its central role in metabolism. We hypothesized that, in the mucopolysacharidoses, interrupted GAG recycling would lead to increased utilization of carbohydrates for normal GAG synthesis and increased lipid use for membrane synthesis. We found generalized deficiencies of lipids, simple carbohydrates, and nucleotides in MPSI mice compared with normal controls. These data, in addition to the increased food intake, reduced surplus of energy, and reduced oxygen consumption (4) provides evidence for increased use of raw material for synthesis. An increase in the biosynthetic compound UDP-glucose is consistent with increased biosynthesis of GAG precursors such as glucuronic acid. We found increases in glucose 6-phosphate and fructose 6-phosphate, which suggest an increase in the importation of simple sugars into the liver. Several lipid metabolites (cholesterol, sphingosine, and glycerolipids) involved in membrane synthesis were elevated, which is consistent with the increased number and size of lysosomes in cells affected with an LSD. More lysosomal membranes are required to accommodate the accumulation of undegraded substrates. In addition, increased plasma membranes are necessary to maintain the integrity of grossly distended cells of the fixed tissue macrophage system, such as Kupffer cells of the liver, retinal pigmented epithelial cells, and microglia of the brain. These data support the hypothesis that impaired recycling coupled with the homeostatic synthesis of GAGs is resulting in metabolite deficiencies.
Interestingly, one mechanism by which substrate reduction therapy exerts its beneficial effects could be linked to this energy imbalance. The benefit of substrate reduction therapy is primarily thought to be from a reduction of the material flowing into the lysosome. It is likely that decreased flow of substrates into the lysosomes reduces the pathology associated with LSDs. However, a secondary benefit could be a reduction in the demand for raw materials necessary for the de novo synthesis of the stored substrates.
Several dipeptides, most amino acids, a number of amino acid-derived metabolites, and urea were elevated in MPSI mice. These data in conjunction with the deficiencies in lipids and carbohydrates suggest that increased protein catabolism, perhaps because of increased autophagy, may be a compensatory mechanism to support intermediary metabolism. To test this hypothesis, we fed MPSI mice a high fat, simple sugar diet for 4 weeks. The metabolic analysis showed a significant reduction toward normal in amino acid derivatives, amino acids, dipeptides, and urea. Further improvement in the levels of simple sugars and some fats provide evidence for an improved ability to meet metabolic demands with less need for protein catabolism. This was likely mediated by an increased importation of energy in the liver as exhibited by further increases in phosphorylated fructose and glucose in dietary-treated animals. Furthermore, we have shown previously that a “Western”-style diet (42% fat) provides MPSI mice with a surplus of energy, beyond cellular demand, that is deposited in adipocytes (4). Thus, MPSI mice appear to be in a state of nutrient deprivation that may be alleviated to some extent by nutritional interventions.
We hypothesized previously that the negative energy balance, as a result of nutrient deprivation, might induce autophagy (4). Consistent with this hypothesis, increased molecular markers of autophagy have been reported in a number of LSDs such as Nieman-Pick type C (10, 11), multiple sulfatase deficiency (12), mucolipidosis Type IV (13–16), Pompe (17), Batten (18), and GM1 gangliosidosis (19). In this study, we hypothesized that the apparent increase in protein turnover was due, in part, to an autophagic process that resulted from the deficiency of simple carbohydrates and lipids. If true, then providing more dietary energy in the form of lipids and simple sugars might reduce the observed level of autophagy in the liver. In both 14-week-old MPSVII mice reared since weaning on the Modified 42% fat diet and MPSI mice treated for 2 weeks with the standard 42% fat diet, the level of lipidated LC3 (LC3II) was reduced (MPSVII, p < 0.039; MPSI, p = 0.080) when compared with untreated MPSVII or MPSI mice. The fact that the MPSI mice did not respond as robustly as the MPSVII mice could be due to the different diets on which the respective groups were maintained. The MPSI mice were maintained on a 22% fat diet and then switched to a 42% fat diet for only 2 weeks prior to analysis. In addition, the 42% fat-fed mice were compared with animals on a 22% fat diet. In contrast, the MPSVII mice were maintained on the Modified 42% fat diet from weaning and were compared with MPSVII mice maintained on a 13% fat diet from weaning. Regardless, these data strongly suggest that one mechanism that contributes to the increased autophagy observed in LSDs is the energy imbalance caused by the sequestration of metabolites in the lysosome. Therefore, MPSI and MPSVII mice appear to suffer from physiologic malnutrition resulting in increased protein degradation and nutrition-sensitive levels of autophagy.
Interestingly, the level of β-actin was consistently increased in both the MPSI and MPSVII animals. Ponceau S staining of the Western blots from both experiments showed that there were comparable levels of total protein between the affected and normal control animals. Although the mechanism is unclear, the increase in β-actin could be in response to the altered cellular architecture in older animals with considerable lysosomal storage accumulation.
In the mucopolysaccharidoses, relatively little is known as to how the genetic deficiency and resulting lysosomal storage leads to the observed clinical phenotypes. Furthermore, the role that metabolic deficiencies and the associated adaptations play in the disease progression is unknown. However, previous data from in vitro cell culture experiments would suggest that a considerable proportion of newly synthesized GAGs are composed of precursors derived from lysosomal degradation (2). Whether the changes observed in isolated cells have an impact on an intact organism was unknown. We showed previously that animals with LSDs have a significant decrease in adiposity because of this energy imbalance (4). We also showed that there were a number of biochemical and transcriptional changes (liver lipoprotein lipase, fatty acid synthase, insulin-like growth factor-binding proteins, cholesterol biosynthesis, etc.) in the livers of MPSVII animals that are consistent with an energy imbalance (9). We show here that the energy imbalance caused by the lack of these lysosome-derived precursors has a major impact on the general metabolic state of the animal and on the level of autophagy.
Based on these observations, we attempted to intervene metabolically with a high fat, simple sugar diet and assess life span, cardiac physiology, and retinal function in MPSVII mice. Dietary intervention failed to improve these functional parameters. However, body weight did improve. The normal response to the Modified 42% fat diet was to have an increased rate of weight gain for the entire experimental period. MPSVII mice responded normally to the increased caloric density of the Modified 42% fat diet with an early increase in the rate of body weight gain. However, as the disease progressed beyond 5 weeks of age, the Modified 42% fat diet did not provide sufficient calories to continue to overcome the energy imbalance. These data show that dietary interventions alone are unlikely to dramatically change disease outcome in MPSVII mice. Interestingly, dietary intervention in CUP-5, the mucolipidosis type IV orthologue in C. elegans, improved metabolism and the survival of individual cells but failed to dramatically increase the life span of the intact animal (13). Although dietary interventions do not address the primary cause of disease, nutritional treatments may aid cellular adaptations to changing conditions and to periods of acute stress. Most likely, dietary treatments may serve an adjunct role in traditional therapies.
Our first attempt at dietary intervention improved several metabolic and biochemical parameters in MPSI and MPSVII mice, yet failed to improve most of the clinical disease in MPSVII animals. Further dietary modifications, such as increasing nucleotide levels, may yet yield functional improvements. The low nucleotide levels may slow the ability of the cell to dynamically react to changing environments (for example, both energy carrier molecules and mRNA turnover). The utility of nutritional modification may be in improving existing therapies by aiding cells in hard to treat organ systems or in aiding patients with residual enzyme activity. Anaplerotic molecules may be particularly effective as targeted adjunct therapies in LSDs (28, 29). Finally, the data from this and previous studies (4, 6, 9) show that MPSI, and many if not most other LSDs, should be viewed both as diseases of abundance but also of physiologic malnutrition.
Supplementary Material
Acknowledgments
We appreciate the helpful discussions with Dr. Anna Cuervo. We are indebted to Dr. Clay Semenkovich for helpful discussions. We thank William Shannon for statistical analysis of the body weight and Jill R. Woloszynek for performing the Kaplan-Meier analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants HD-0055461 and NS-43205 (to M. S. S.) and DC-004665, NS-057105, and DC-08321 (to K. K. O.). This work was also supported by a grant from the Mucopolysaccharidosis Society (to J. C. W. and M. S. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
- LSD
- lysosomal storage disease
- GAG
- glycosaminoglycan
- MPS
- mucopolysaccharidosis
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1.Vellodi A. (2005) Br. J. Haematol. 128, 413–431 [DOI] [PubMed] [Google Scholar]
- 2.Rome L. H., Hill D. F. (1986) Biochem. J. 235, 707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walkley S. (2009) J. Inherit. Metab. Dis. 32, 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woloszynek J. C., Coleman T., Semenkovich C. F., Sands M. S. (2007) J. Biol. Chem. 282, 35765–35771 [DOI] [PubMed] [Google Scholar]
- 5.Fratantoni J. C., Hall C. W., Neufeld E. F. (1968) Science 162, 570–572 [DOI] [PubMed] [Google Scholar]
- 6.Beamer W., Coleman D. (1982) Mouse Newsletter 67, 21 [Google Scholar]
- 7.Du H., Heur M., Duanmu M., Grabowski G. A., Hui D. Y., Witte D. P., Mishra J. (2001) J. Lipid Res. 42, 489–500 [PubMed] [Google Scholar]
- 8.Venugopal B., Browning M. F., Curcio-Morelli C., Varro A., Michaud N., Nanthakumar N., Walkley S. U., Pickel J., Slaugenhaupt S. A. (2007) Am. J. Hum. Genet. 81, 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woloszynek J. C., Roberts M., Coleman T., Vogler C., Sly W., Semenkovich C. F., Sands M. S. (2004) Biochem. J. 379, 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko D., Milenkovic L., Beier S., Manuel H., Buchanan J., Scott M. (2005) PLoS Genet. 1, 81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacheco C. D., Elrick M. J., Lieberman A. P. (2009) Hum. Mol. Genet. 18, 956–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Settembre C., Fraldi A., Jahreiss L., Spampanato C., Venturi C., Medina D., de Pablo R., Tacchetti C., Rubinsztein D. C., Ballabio A. (2008) Hum. Mol. Genet. 17, 119–129 [DOI] [PubMed] [Google Scholar]
- 13.Schaheen L., Dang H., Fares H. (2006) Dev. Biol. 293, 382–391 [DOI] [PubMed] [Google Scholar]
- 14.Venugopal B., Mesires N. T., Kennedy J. C., Curcio-Morelli C., Laplante J. M., Dice J. F., Slaugenhaupt S. A. (2009) J. Cell. Physiol. 219, 344–353 [DOI] [PubMed] [Google Scholar]
- 15.Venkatachalam K., Long A. A., Elsaesser R., Nikolaeva D., Broadie K., Montell C. (2008) Cell 135, 838–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergarajauregui S., Connelly P. S., Daniels M. P., Puertollano R. (2008) Hum. Mol. Genet. 17, 2723–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda T., Ewan L., Bauer M., Mattaliano R. J., Zaal K., Ralston E., Plotz P. H., Raben N. (2006) Ann. Neurol. 59, 700–708 [DOI] [PubMed] [Google Scholar]
- 18.Koike M., Shibata M., Waguri S., Yoshimura K., Tanida I., Kominami E., Gotow T., Peters C., von Figura K., Mizushima N., Saftig P., Uchiyama Y. (2005) Am. J. Pathol. 167, 1713–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takamura A., Higaki K., Kajimaki K., Otsuka S., Ninomiya H., Matsuda J., Ohno K., Suzuki Y., Nanba E. (2008) Biochem. Biophys. Res. Commun. 367, 616–622 [DOI] [PubMed] [Google Scholar]
- 20.Galvin N., Vogler C., Levy B., Kovacs A., Griffey M., Sands M. S. (2008) Pediatr. Dev. Pathol. 11, 185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heldermon C. D., Hennig A. K., Ohlemiller K. K., Ogilvie J. M., Herzog E. D., Breidenbach A., Vogler C., Wozniak D. F., Sands M. S. (2007) PLoS ONE 2, e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Vusse G. J., Glatz J. F., Stam H. C., Reneman R. S. (1992) Physiol. Rev. 72, 881–940 [DOI] [PubMed] [Google Scholar]
- 23.Wang W., Lopaschuk G. D. (2007) Expert Rev. Cardiovasc. Ther. 5, 1123–1134 [DOI] [PubMed] [Google Scholar]
- 24.Horiuchi M., Yoshida H., Kobayashi K., Kuriwaki K., Yoshimine K., Tomomura M., Koizumi T., Nikaido H., Hayakawa J., Kuwajima M., Saheki T. (1993) FEBS Lett. 326, 267–271 [DOI] [PubMed] [Google Scholar]
- 25.Schuldt A. J., Hampton T. J., Chu V., Vogler C. A., Galvin N., Lessard M. D., Barker J. E. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumagai A. K. (1999) Diabetes Metab. Res. Rev. 15, 261–273 [DOI] [PubMed] [Google Scholar]
- 27.Ohlemiller K. K., Vogler C. A., Roberts M., Galvin N., Sands M. S. (2000) Exp. Eye Res. 71, 469–481 [DOI] [PubMed] [Google Scholar]
- 28.Roe C. R., Mochel F. (2006) J. Inherit. Metab. Dis. 29, 332–340 [DOI] [PubMed] [Google Scholar]
- 29.Roe C. R., Sweetman L., Roe D. S., David F., Brunengraber H. (2002) J. Clin. Invest. 110, 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.