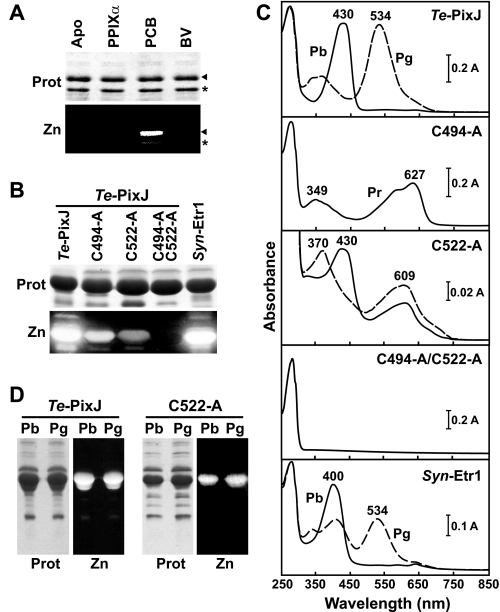
FIGURE 2.
Assembly of Te-PixJ and Syn-Etr1 with PCB. A, Te-PixJ specifically binds PCB. E. coli extracts containing His6-tagged wild-type Te-PixJ(Cyc-GAF) apoprotein were incubated with protoporphyrin IXα (PPIXα), BV, or PCB. The Te-PixJ(Cyc-GAF) polypeptide was then purified by Ni-NTA chromatography, subjected to SDS-PAGE, and either stained for protein with Coomassie Blue (Prot) or assayed for the bound bilin by zinc-induced fluorescence (Zn). Apo, apoprotein before incubation with the bilins. The arrow indicates the full-length Te-PixJ(Cyc-GAF) polypeptide, whereas the asterisk indicates a likely N-terminal degradation product that binds chromophore. B, covalent binding of PCB with wild-type Syn-Etr1(Cyc-GAF) and with Te-PixJ(Cyc-GAF) cysteine mutants affected in the bilin attachment sites. The apoproteins were co-expressed with the pair of enzymes needed to synthesize PCB and then purified by Ni-NTA chromatography. SDS-PAGE analyses were as described in panel A. C, absorption spectra of samples in panel B either kept in the dark (Pb and Pr, solid lines) or following saturating blue light (Pg, dashed lines). D, purified Te-PixJ(Cyc-GAF) and the C522A chromoprotein were either irradiated with blue light (430 nm) to convert to the green-absorbing state (Pg) or left in the dark-adapted blue-absorbing state (Pb). The samples were then heated in SDS-PAGE buffer and subjected to denaturing electrophoresis. The resultant SDS-PAGE gel was either stained for protein with Coomassie Blue (Prot) or assayed for the bound bilin by zinc-induced fluorescence under UV light (Zn).