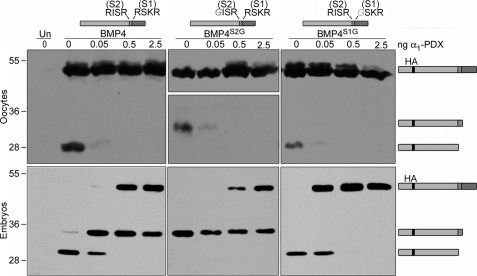
FIGURE 3.
An α1-PDX-insensitive enzyme acts redundantly with furin and PC6 to cleave the optimal site of BMP4 in embryos but not oocytes. Western blot of oocyte lysates (top panels) or blastocoel fluid from embryos (bottom panels) not injected (Un) or injected with RNA encoding wild type or cleavage mutant forms of pro-BMP4 (illustrated above each panel) either alone or together with increasing doses of α1-PDX are as indicated. Blots were probed with HA-specific antibodies to detect precursor proteins and cleaved prodomain fragments (illustrated schematically to right of gel). Monomeric forms of pro-BMP4 are present at significantly higher levels than cleavage products in oocyte lysates, and thus when Western blots are exposed for sufficient periods of time to visualize cleavage products, bands corresponding to precursor proteins are overexposed. This is particularly true in the case of BMP4S2G, because cleavage products are unstable, and thus two different exposures of the same Western blot are shown. Results were reproduced in at least three experiments.