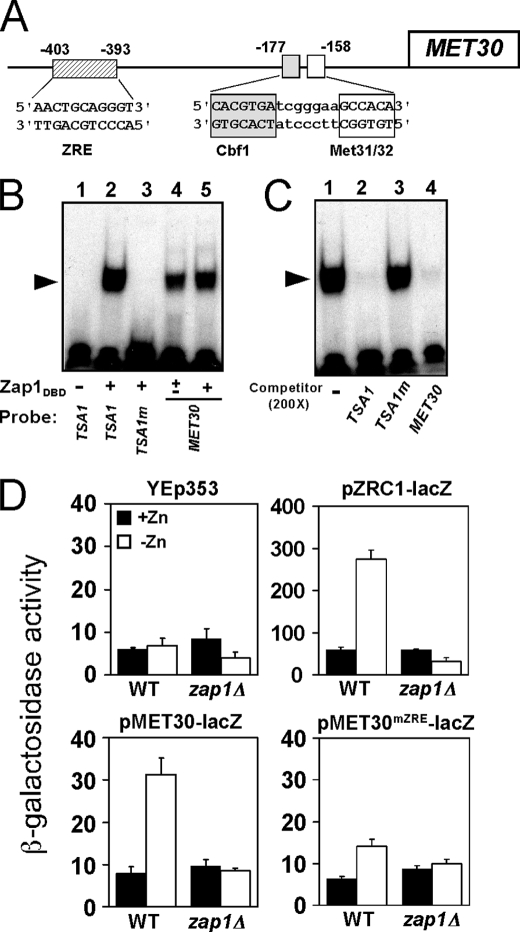
FIGURE 4.
MET30 is a Zap1 direct target. A, diagram of the MET30 promoter. Met4 is recruited to this promoter by binding to the Cbf1, Met31, and Met32 proteins bound at the indicated sites. A potential ZRE is located upstream of these sites of Met4 recruitment. B, Zap1 binds specifically to the MET30 ZRE in vitro as assessed by electrophoretic mobility shift assay. Radiolabeled double-stranded oligonucleotides (0.5 pmol, 10,000 cpm) containing ZRE-like sequences from the indicated promoters were used as probes. The probes were mixed with 0 (−), 0.2 (±) or 0.4 μg (+) per reaction of purified Zap1 DNA binding domain (Zap1DBD). FP denotes the free probe, and the arrow indicates the Zap1DBD-DNA complex. The wild type TSA1 ZRE and mutant TSA1 ZRE (TSA1m) were included as positive and negative controls, respectively. The MET30 ZRE was used as probe in lanes 4 and 5. C, electrophoretic mobility shift assays were performed using the wild type TSA1 ZRE as the probe. Nonradiolabeled oligonucleotides were used as competitors at a 200-fold excess of the labeled probe concentration. Wild type TSA1 ZRE and MET30 ZRE fragments competed effectively for Zap1 binding, whereas the mutant TSA1m ZRE did not. D, MET30 ZRE is required for induction of gene expression in low zinc in vivo. Wild type (WT) or zap1Δ mutant cells bearing the pMET30-lacZ promoter fusion or the MET30 promoter fusion with a mutated ZRE (pMET30mZRE-lacZ) were grown in high zinc (+Zn, LZM + 1000 μm ZnCl2) and low zinc (−Zn, LZM + 1 μm ZnCl2) for 14–20 h. Cells were then harvested and assayed for β-galactosidase activity. The vector YEp353 was used as negative control, and the Zap1-responsive pZRC1-lacZ served as a positive control. Shown are the means from three independent experiments, and the error bars indicate ± S.D.