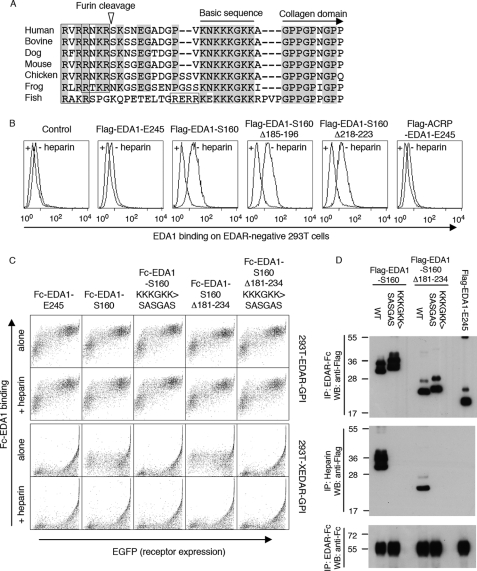
FIGURE 8.
EDA1 contains a heparan sulfate proteoglycan binding sequence in front of the collagen domain. Panel A, sequence alignment around the mature N terminus of EDA1. Conserved residues are highlighted. The basic sequence responsible for HSPG binding, and the collagen domain are indicated, furin consensus sites are boxed, and the furin cleavage site (of human EDA) is shown with an arrowhead. Species analyzed are Homo sapiens, Bos taurus, Canis familiaris, Mus musculus, Gallus gallus, Xenopus tropicalis, and Gasterosteus aculeatus. Panel B, untransfected, EDAR-negative 293T cells were stained with the indicated Flag-tagged versions of EDA1 in the presence or absence of heparin, and analyzed by FACS. Panel C, 293T cells were transfected with GPI-anchored extracellular domains of EDAR or XEDAR, together with an EGFP tracer, and then stained with the indicated Fc-tagged versions of EDA1, in the presence or absence of heparin. Cells were analyzed by 2-color FACS. Panel D, indicated Flag-tagged EDA1 constructs were pulled down with either EDAR-Fc (upper panel) or with heparin-Sepharose (middle panel), and detected by Western blot with an anti-Flag antibody. Immunoprecipitated EDAR-Fc was revealed with an anti-human IgG antibody (lower panel). All EDA1 constructs bound to EDAR-Fc, but only those with an intact proteoglycan-binding region interacted with heparin.