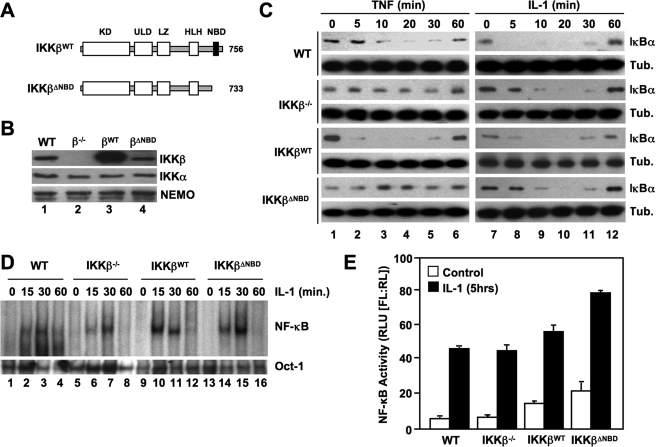
FIGURE 1.
The IKKβ NBD is required for TNF- but not IL-1-induced classical NF-κB activation. A, the structural domains of wild-type IKKβ (IKKβWT) and IKKβΔNBD are shown. KD, kinase domain; LZ, leucine zipper; ULD, ubiquitin-like domain; HLH, helix-loop-helix. IKKβΔNBD is a truncation mutant encompassing residues 1–733 that lacks the C-terminal 23 amino acids containing the NBD (13, 14). B, lysates from WT, IKKβ−/− (β−/−), IKKβWT (βWT), and IKKβΔNBD (βΔNBD) MEFs were immunoblotted using the antibodies indicated (right). C, WT, IKKβ−/−, IKKβWT, and IKKβΔNBD MEFs were incubated with either TNF (10 ng/ml) (left) or IL-1α (10 ng/ml) (right) for the times indicated, and then lysates were immunoblotted using anti-IκBα or anti-tubulin (Tub.) as a loading control. D, the same panel of MEFs was treated with IL-1α for the indicated times, and then nuclear extracts were prepared for EMSA. Assays were performed using either a consensus NF-κB binding site probe (top) or an Oct-1 probe as a loading control (bottom). E, MEFs were transiently transfected with the NF-κB-dependent reporter pBIIx-firefly luciferase together with β-actin Renilla luciferase. Twenty-four hours later, cells were either left untreated or treated for a further 5 h with IL-1α, and then NF-κB activity was determined by a dual luciferase assay.