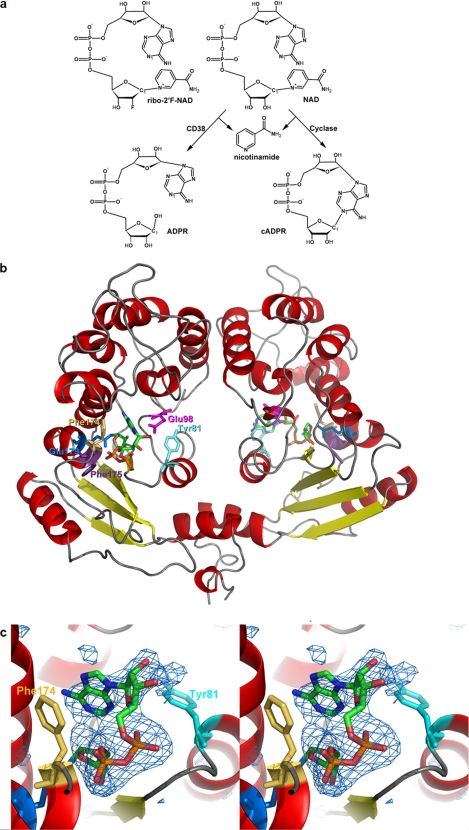
FIGURE 1.
Crystal structure of the complex of cyclase with ribo-2′F-NAD. a, chemical structure of the substrate ribo-2′F-NAD and the reactions catalyzed by CD38 and the cylase. b, crystal structure of the cyclase dimer with the intermediates at each of the active sites of the monomer. The color scheme for the secondary structures is: red, α-helix; yellow, β-sheet; gray, coil. The color scheme for the residues is: cyan, Tyr-81; beige, Phe-174; blue, Glu-179; magenta, Glu-98; purple, Phe-175. The intermediates are colored by their elements: green, carbon; red, oxygen; orange, phosphorus; blue, nitrogen; light green, fluorine. c, stereo view of the folded conformation with electron density from an omit Fo − Fc map contoured at 2.7 σ and shown as blue wire mesh. Other color schemes are the same as in b. The average B-factor is 76 Å2 for the folded intermediate.