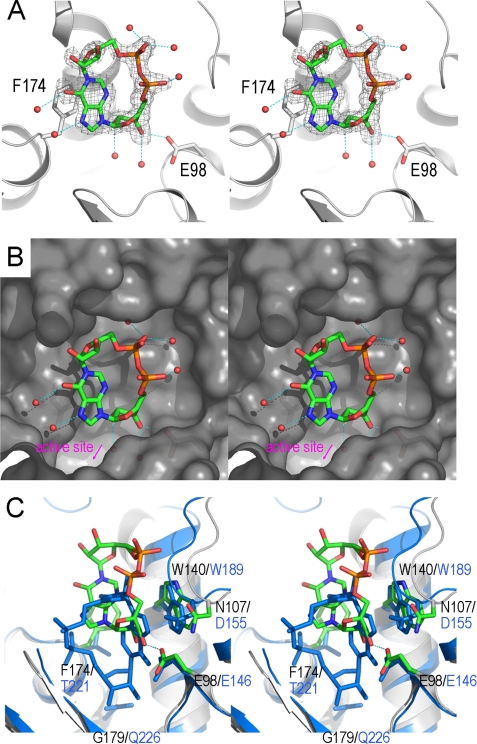
FIGURE 4.
Divergent binding of ADPRC and CD38 with a cADPR analog, N1-cIDPR. A, the structure of the binding site of the E179GADPRC·N1-cIDPR complex. The color scheme for residues involved in N1-cIDPR binding is the same as in Fig. 2A. Residues Glu-98 and Phe-174 have polar and non-polar interactions with N1-cIDPR, respectively, and play a major role in N1-cIDPR binding. Red spheres are water molecules that participate in N1-cIDPR binding through hydrogen-bonding interactions. Electron density for N1-cIDPR is shown as gray isomesh contoured at 2.5 σ. B, surface representation, colored in gray, of the N1-cIDPR binding site with N1-cIDPR (sticks) and water molecules (spheres) in the binding cleft. C, N1-cIDPR binds to a site in ADPRC that is different from CD38. In CD38 (aquamarine), N1-cIDPR binds deep into the active site, whereas in ADPRC, the site is farther away from the catalytic residue (6.1Å) and in an upside-down and left-right conformation.