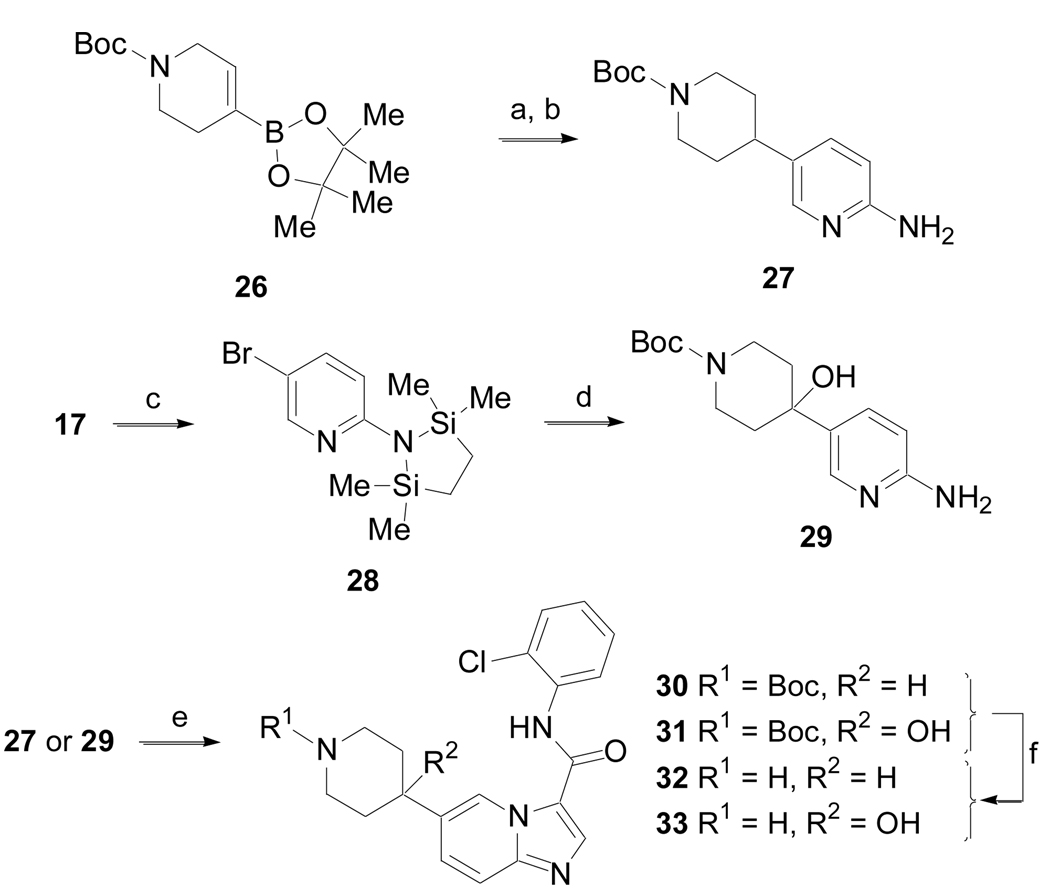
Scheme 6.
(Method F). (a) 17, Pd(PPh3)4, Na2CO3, CH3CN, H2O, 90 °C, 6 h; (b) 10% Pd/C, H2 (1 atm), EtOH, 16 h, 67% for two steps; (c) n-BuLi (2 equiv), (Me2SiClCH2)2, THF, − 78 °C, 1 h; (d) n-BuLi (1 equiv), N-Boc-4-piperidone, − 78 °C to rt, 18 h, 58%; e) DMF-DMA, 85 °C, toluene 8 h, then BrCH2C(O)NH-2-Cl-Ph, MeOH, 85 °C, 18 h, 26 – 30%; f) 30% TFA in DCM, 1 h, rt, 82%.