Table 1.
IC50 determinations for inhibiting EphB3 phosphorylation of BTK-peptide
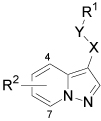 | ||||||
|---|---|---|---|---|---|---|
| Cmpd | X | Y | R1 | R2 | Method | IC50 (µM) |
| 1 | C=O | NH | 2-Cl-Ph | H | --- | 1.0 |
| 34 | C=O | NH | Ph | H | A | NA |
| 35 | C=O | NH | 2-F-Ph | H | A | ~ 20 |
| 36 | C=O | NH | 3-Cl-Ph | H | A | NA |
| 37 | C=O | NH | 4-Cl-Ph | H | A | NA |
| 38 | C=O | NH | 2,3-Cl2-Ph | H | A | ~20 |
| 39 | C=O | NH | 2-OMe-Ph | H | A | NA |
| 40 | C=O | NH | 2-CF3-Ph | H | A | > 20 |
| 41 | C=O | NH | 2-CN-Ph | H | A | > 20 |
| 42 | C=O | NH | 2-MeSO2Ph | H | A | NA |
| 43 | C=O | NMe | 2-Cl-Ph | H | A | NA |
| 44 | C=O | NH | CH2-2-Cl-Ph | H | A | NA |
| 7 | NH | C=O | 2-Cl-Ph | H | B | > 20 |
| 45 | CH2 | NH | 2-Cl-Ph | H | A | > 20 |
| 46 | C=O | NH | 2-Cl-Ph | 4-Me | C | ~ 15 |
| 47 | C=O | NH | 2-Cl-Ph | 5-Me | C | 0.26 |
| 48 | C=O | NH | 2-Cl-Ph | 6-Me | C | ~ 15 |
| 49 | C=O | NH | 2-Cl-Ph | 7-Me | C | NA |
| 50 | C=O | NH | 2-Cl-Ph | 4-Cl | C | ~ 20 |
| 51 | C=O | NH | 2-Cl-Ph | 5-Cl | C | 2.0 |
| 52 | C=O | NH | 2-Cl-Ph | 6-Cl | C | > 20 |
| 53 | C=O | NH | 2-Cl-Ph | 5-OMe | C | 0.19 |
| 54 | C=O | NH | 2-Cl-Ph | 5-Ph | C | 0.066 |
| 55 | C=O | NH | 2-Cl-Ph | 5-NMe2 | C | 0.077 |
| 56 | C=O | NH | 2-Cl-Ph | 5-Pyrr | D | 0.20 |
| 57 | C=O | NH | 2-Cl-Ph | 5-Morph | D | 0.24 |
| 58 | C=O | NH | 2-Cl-Ph | 5-Pip | D | 0.063 |
| 59 | C=O | NH | 2-Cl-Ph | 5-OBn | C | ~ 20 |
NA: Not active at 20 µM; Pyrr = N-pyrrolidinyl; Morph = N-morpholinyl; Pip = N-piperidinyl
