Figure 4.
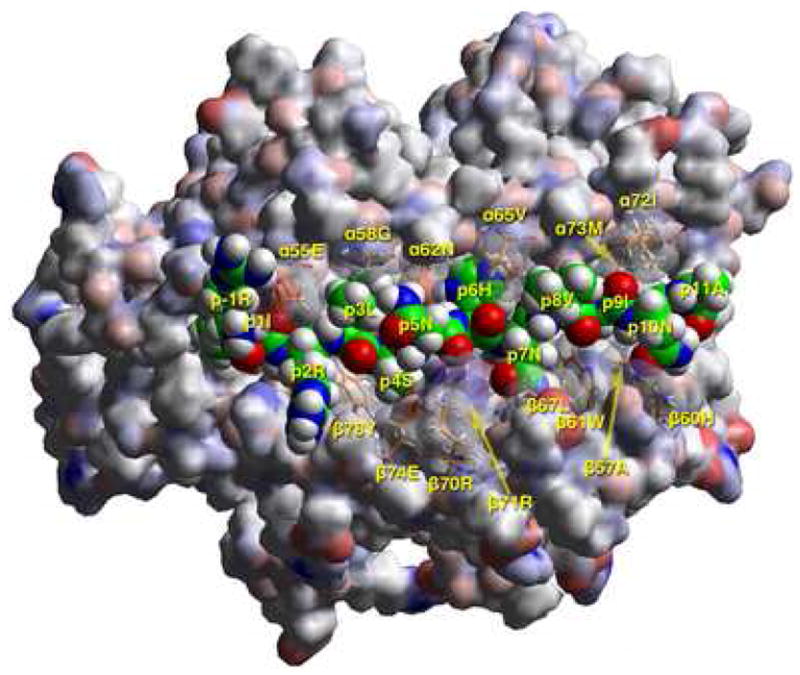
A) T cell receptor view of the antigenic peptide (RIRLSNHNVINA, anchors in bold) in the groove of DRB1*1401 (DR1401), after energy minimization at pH 5.4 based on the crystal structure of HLA-DRB1*0301 in complex with the CLIP peptide (13). The αlβl domain of the DR14 molecule is depicted in van der Waals surface representation, with the surface atomic charges color-coded (blue, positive; gray, neutral; red, negative), and the antigenic peptide is shown in space filling form (color code: carbon, green; oxygen, red; nitrogen, blue; hydrogen, white; sulfur, yellow). Several DR14 residues that have particular interactions with the antigenic peptide (α55Glu, α58Gly, α62Asn, α65Val, α72Ile, α73Met, β57Ala, β60His, β61Trp, β67Leu, β70Arg, β71Arg, β74Glu, β78Tyr) are shown in stick form with the same color-code as the antigenic peptide with the exception of carbon (orange). P1Ile is in pocket 1, p6His+ in pocket 6, and p9Ile in pocket 9 (first and third pointing into the plane of the paper, and the second only partly so) as expected of a peptide bound to DR14 with high affinity. Figure drawn with the aid of WebLabViewer version 3.5 and DSViewer Pro version 6.0, of Accelrys.
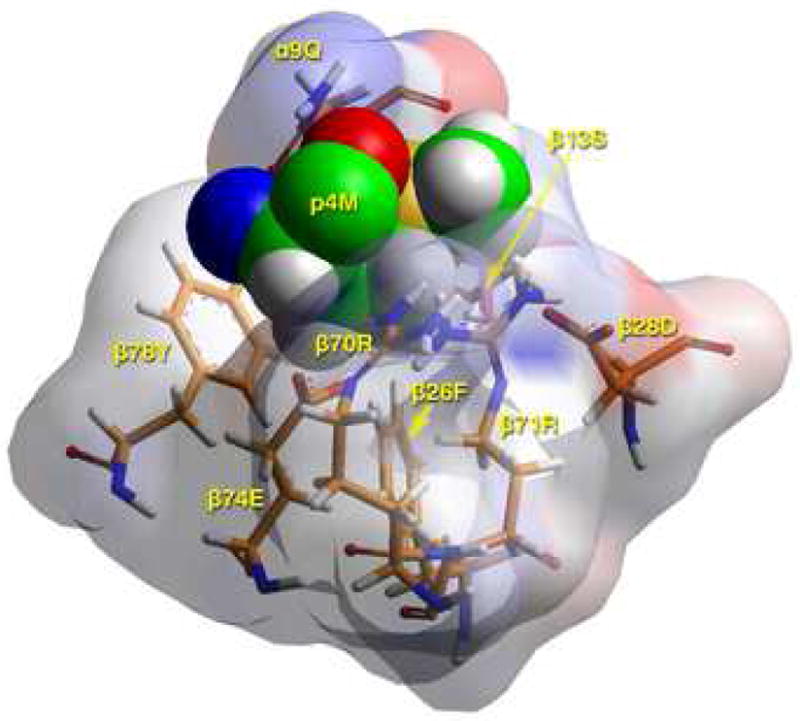
B) TCR view of pocket 4 of the complex of the peptide RIRLSNHNVINA with the HLA-DR14 allele. The arrangement of the various αβ residues in this pocket promotes the anchoring of both small and large aliphatic, but also polar and some charged amino acids at p4. The combination of β74Glu/β70Arg/β28Asp/β71Arg allows Asp and His binding (but not Arg/Lys) in this pocket. Likewise, the presence of β13Ser, β26Phe and α9Gln leaves sufficient room for anchoring of bulky aliphatic (but not aromatic) residues in this pocket. The figure has been rotated by 20° with respect to the y-axis (left-hand side into the plane of the paper, right-hand side above the plane of the paper) so that the interactions between various residues could be fully exposed. Color and depiction conventions identical to those in Figure 4A. Figure drawn with the aid of WebLabViewer version 3.5 and DSViewer Pro version 6.0, of Accelrys.
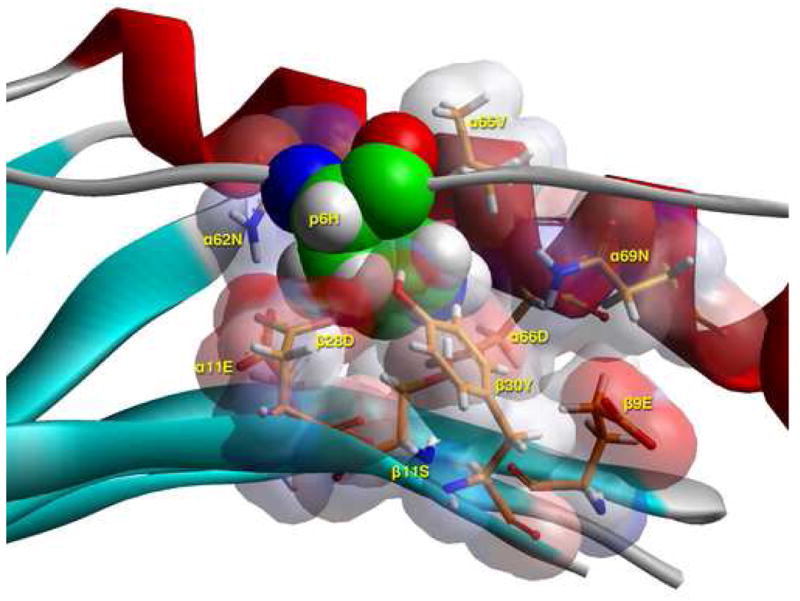
C) Side view of pocket 6 of the complex of the peptide RIRLSNHNVINA with the HLA-DR14 allele. This is seen from the Cα of β57Ala at the level of the β-sheet floor. The arrangement of the various αβ residues in this pocket, and the ability of β9Glu to be flexible, promotes the anchoring of a wide variety of residues (from positively charged Arg, His (shown here), to polar Ser, Gln and small and large aliphatics), but no acidic ones or Tyr. The presence of β11Ser provides sufficient space for the larger of the allowed residues, while the presence of four acidic residues (α11Glu, α66Asp, β9Glu, β28Asp) provides a suitable environment for the binding of positively charged residues in this pocket, excluding negatively charged ones. For orientation purposes we also show part of the α1 helix (residues 62 to 69), the β–sheet floor from where residues originate, and the peptide backbone to which p6Asp belongs. In all probability p6His would be charged even at the extracellular pH of 7.4 (energy minimisation performed at pH 5.4, the pH of the binding study) by induction, due to its proximity to α66Asp. Color and depiction conventions identical to those in Figure 4A. In addition, α-helix is in red, β-sheet is in turquoise and all other secondary structure forms (random coil, polyproline type II helix of the antigenic peptide are in gray). Figure drawn with the aid of WebLabViewer version 3.5 and DSViewer Pro version 6.0, of Accelrys.
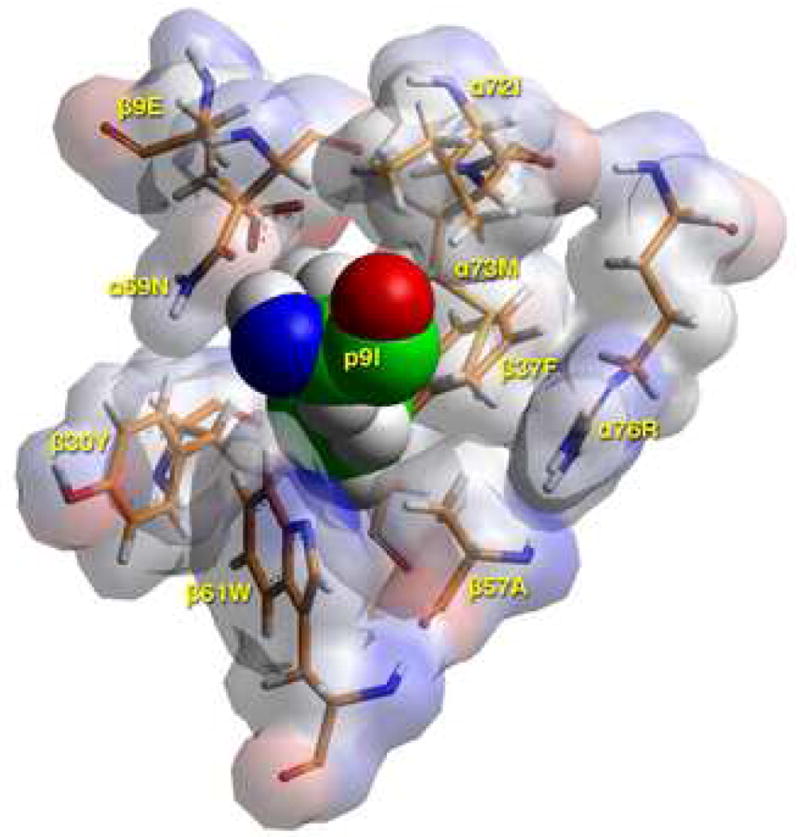
D) TCR view of pocket 9 of the complex of the peptide RIRLSNHNVINA with the HLA-DR14 allele. The arrangement of the various αβ residues in this pocket promotes the anchoring of either large aliphatic/aromatic amino acids (but hardly tryptophan) at p9. The presence of β30Tyr limits the space available, while the combination α72Ile/α73Met/β37Phe/β57Ala/α76Arg favors this preference, excluding acidic and basic amino acids from this pocket. Contrast this to the situation in the H2-Ek allele (α72Val/α73Met/β9Glu/β30Tyr/β37Asn/α57Asp/α76Arg), where Arg/Lys are exclusively favored at this pocket. There, the b9Glu/b37Asn combination attracts the positively charged residues, while the β57Asp—α76Arg salt bridge neutralizes the electrostatic repulsion of the latter residue for basic p9 residues as they approach the mouth of the pocket (16). Color and depiction conventions identical to those in Figure 4A. Figure drawn with the aid of WebLabViewer version 3.5 and DSViewer Pro version 6.0, of Accelrys.
