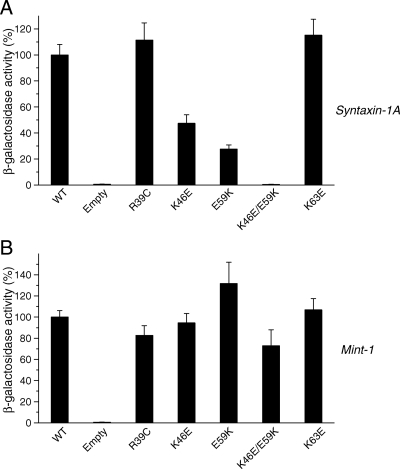
Figure 5.
Mutations in domain 1 of Munc18-1 result in dramatic reductions in the binding to syntaxin-1 but not to mint-1. Mutations were introduced in the residues in domain 1 of Munc18-1 that are involved in the binding to the ‘closed’ conformation of syntaxin-1 (Misura et al., 2000). The binding between these Munc18-1 mutants and syntaxin-1A or mint-1 was analyzed with yeast two-hybrid assays. In each assay, β-galactosidase activities of the transformed yeast clones were quantified (see Materials and Methods) and normalized so that the activity of the yeast clones transformed with the wild-type Munc18-1 was set to 100%. Error bars indicate SEM (n = 12–14 for binding to syntaxin-1; n = 8–12 for binding to mint-1). Note that K46E/E59K mutation in Munc18-1 abolished the binding to syntaxin-1 but not to mint-1.