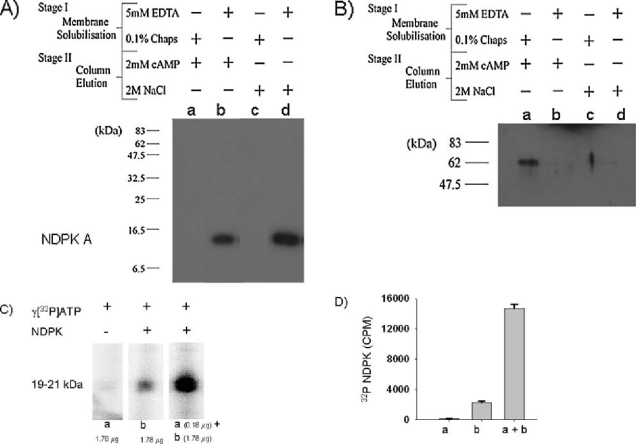
Fig. 3.
Panel (A) Western blot of NDPK A from ovine airway membrane fractions prepared in two sequential stages, each involving two different methods of ‘Membrane Solubilisation’ and ‘Column Elution’. Stage I Membrane Solubilisation: Lanes (a, b, c, d) contain proteins extracted from post-nuclear ovine airway membranes using either 0.1% Chaps (a & c) or 5 mM EDTA (b & d). Stage II Protein Isolation: Solubilised proteins from stage I were applied to a cAMP-POROS 20 EP affinity column (generated according to manufacturer’s instruction). After low salt washes, column-retained proteins were eluted with either 2 mM cAMP (fractions a, b) or 2M salt (fractions c, d). The fractions were run on SDS-PAGE, blotted onto PVDF and probed with an anti-NDPK A antibody. NDPK A was only found in lanes b & d. Panel (B)Western blot of NDPK A shows that overlay of the blot (panel A) with a solution containing NDPK A (lane b, 25 (g) results in NDPK A binding to a 62 kDa protein only in lane a (eluted with cAMP from the cAMP-affinity column). Panel (C) Enhanced phosphorylation of NDPK by eluate from cAMP-affinity column. Incubation of fractions containing NDPK A (Panel A, lane b, 1.78 µg protein) with fractions containing the 62 kDa protein (Panel B, lane a, 10× dilution), in the presence of γ[32P]-ATP and Mn2+, results in a four fold enhancement in NDPK phosphorylation. Quantitation is shown in panel D (n = 3).