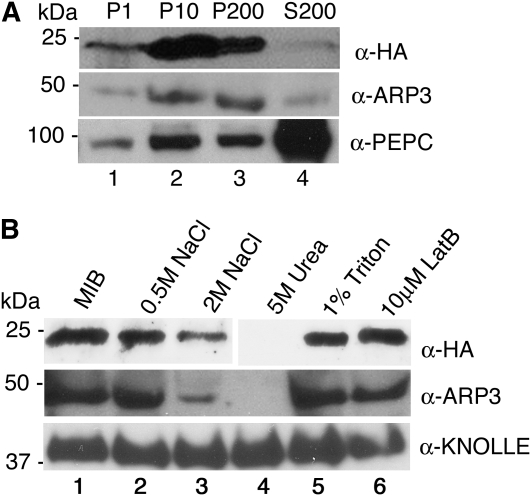
Figure 6.
The ARP2/3 protein complex is peripherally associated with membrane fractions. A, Plant protein extracts were centrifuged sequentially at different speeds: 1,000g (P1; lane 1), 10,000g (P10; lane 2), and 200,000g to generate final pellet (P200; lane 3) and soluble cytosolic (S200; lane 4) fractions. In each case, the cell fractions were loaded by equal proportion. The fractions from arpc4-t2 ARPC4:HA and wild-type plants were probed with anti-HA (top panel) and anti-ARP3 (middle panel) antibodies, respectively. PEPC, an abundantly soluble protein (bottom panel), was used as an internal control to monitor the soluble protein fractions. B, ARP2/3 is a peripheral membrane complex. Microsomal pellets were resuspended in MIB alone (control; lane 1) or with MIB supplemented with increasing NaCl concentrations (lanes 2 and 3), 5 m urea (lane 4), 1% Triton (lane 5), and the actin filament-depolymerizing drug latrunculin B (LatB; lane 6). After incubation for 30 min under the different conditions, the soluble and membrane-associated fractions were separated by ultracentrifugation and pellets were probed with anti-HA (top panel), anti-ARP3 (middle panel), and anti-KNOLLE to detect the integral membrane syntaxin (bottom panel).