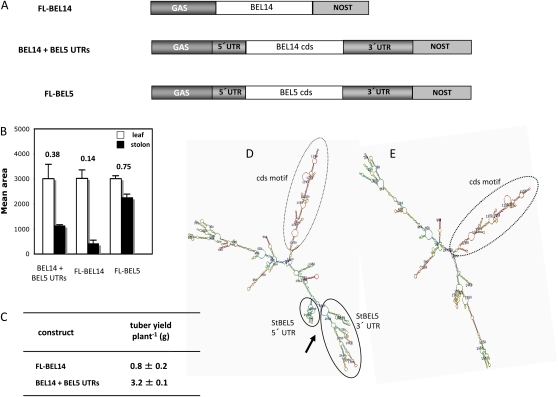
Figure 4.
A, Three RNA constructs driven by the leaf-abundant GAS promoter were tested for their capacity to move from leaf veins to stolon tips: FL-BEL14 (contains BEL14 cds plus both native BEL14 UTRs), BEL14 + both StBEL5 UTRs replacing the StBEL14 UTRs, and full-length StBEL5. StBEL14 was chosen as a test RNA because it is not abundant in stems or stolons (Chen et al., 2003). B, Relative RNA accumulation in new leaves and 0.5-cm samples from the tip of the stolon was quantified in transgenic lines expressing these chimeric transcripts. Transgenic plants were grown under short days (8 h of light/16 h of dark) for 12 d, and the RNA was extracted from new leaves (white bars) and from 0.5-cm stolon tips (black bars) from three separate plants for each construct. C, At the same time, tuber yields were scored for BEL14 lines with and without UTR fusions. Homogenous RT-PCR products were quantified using ImageJ software (Abramoff et al., 2004) and normalized using rRNA values. se values of three clones from one independent transgenic line per construct are shown. A movement factor is provided for mobility assays above each set of constructs (B). The movement factor is equal to the relative stolon tip RNA quantity divided by the relative leaf RNA quantity. D and E, Representative Mfold models of the two BEL14 RNA constructs assayed for movement. Models for the RNA structure of BEL14 + the BEL5 UTRs exhibited the same multifingered structure (D, arrow) formed by the UTRs (D, solid circles) of the full-length StBEL5 transcript (Fig. 3A, full-length model, solid circles). All predicted Mfold models for StBEL14 exhibited a stable, conserved motif arising from its cds (D and E, dotted circles). NOST, Nopaline synthase terminator. [See online article for color version of this figure.]