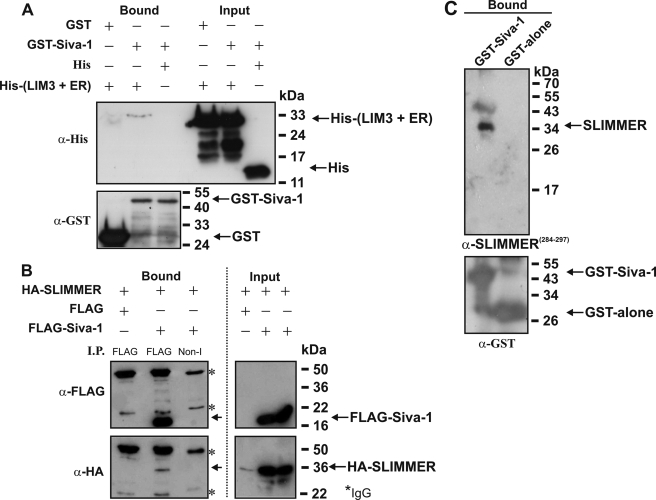
FIGURE 3.
Siva-1 binds SLIMMER. A, GST-Siva-1 and His-tagged LIM domain 3 + ER of SLIMMER (His-(LIM3 + ER)) were co-expressed in E. coli. GST-proteins (5 μg) and associated proteins were bound to glutathione-Sepharose, washed extensively with Tris-buffered saline with 1% Triton X-100, and eluted with SDS-PAGE reducing buffer. Eluted proteins (Bound) and whole-cell extracts (Input) were immunoblotted for recombinant GST (lower panel) or His protein (upper panel) expression. Western blots are representative of three experiments. B, HA-SLIMMER and FLAG-Siva-1 or FLAG-vector were co-transfected into COS-1 cells, and lysates (∼1 mg) prepared in Tris lysis buffer with 1% Triton X-100 were immunoprecipitated (I.P.) with non-immune (Non-I) or anti-FLAG antibodies, washed in Tris-buffered saline without detergent, and immunoblotted with anti-FLAG to confirm FLAG-Siva-1 immunoprecipitation (upper left). Co-immunoprecipitation of HA-SLIMMER specifically with FLAG-Siva-1 was confirmed by immunoblotting with anti-HA (lower left). Protein expression shown by anti-FLAG (upper right) or anti-HA (lower right) immunoblots. Asterisks indicate IgG heavy and light chains. Blots are representative of three independent experiments. Input lanes were loaded with ∼25 μg of protein. C, GST-Siva-1 pulldown of endogenous SLIMMER from skeletal muscle lysates. GST-Siva-1 or GST-alone was expressed in E. coli and ∼500 μg of lysate, purified using glutathione-Sepharose. GST-Siva-1 or GST-alone bound to glutathione-Sepharose was incubated with murine skeletal muscle lysate, the Sepharose beads were washed extensively in Tris-buffered saline without detergent, and bound protein was eluted with SDS-PAGE reducing buffer. Samples were separated on 12.5% SDS-PAGE and immunoblotted with antibodies specific for SLIMMER (α-SLIMMER284–297) (upper panel) or the GST-tag (lower panel).