Figure 1.
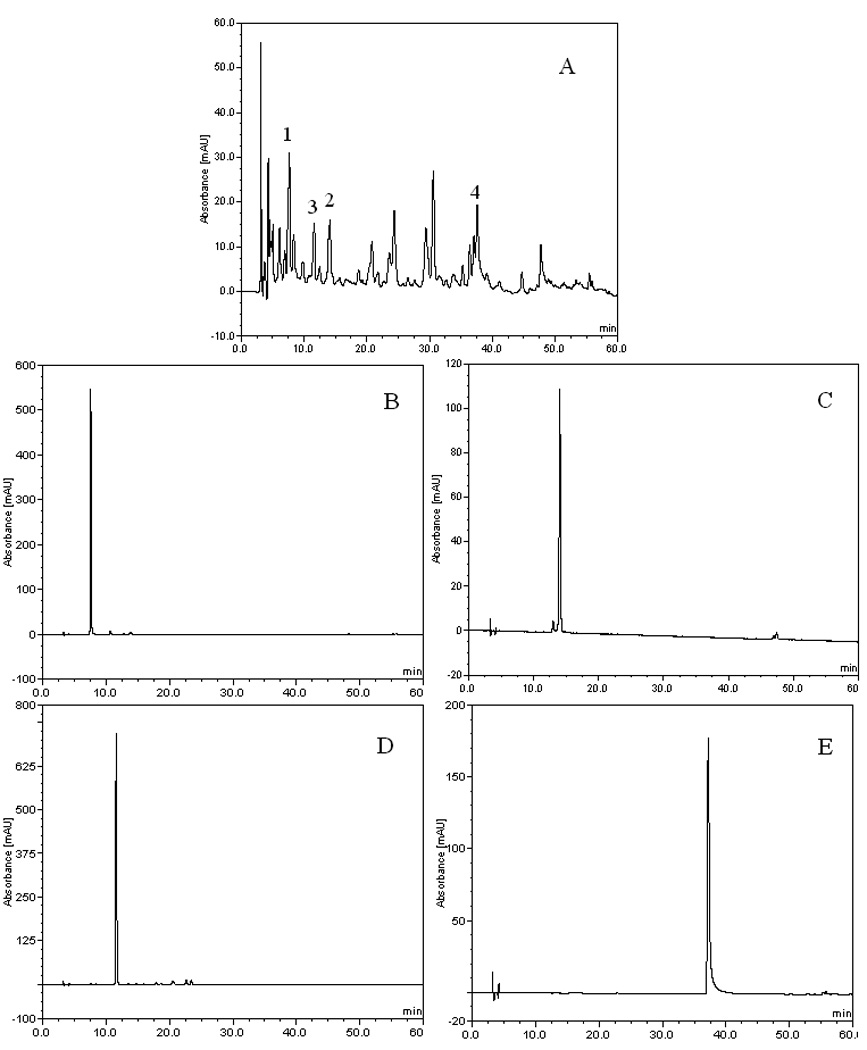
HPLC chromatogram of the acetic ether fraction and HSCCC fractions from black currant. Column: inertsil ODS-SP column (4.6 mm × 250 mm, 5 µm); the mobile phase A: acetonitrile, B: methanol, C: 0.2% formic acid, the gradient was as follows: A-B-C (7.5:7.5:85, v/v ) to A-B-C (17.5:17.5:65, v/v ) in 40 min, then to A-B-C (38:37:25, v/v ) in 20 min. detection: 254 nm; flow rate: 1.0 mL/min. (A) acetic ether fraction from black currant, (B) protocatechuic acid (1), (C) caffeic acid (2), (D) 4-hydroxybenzoic acid (3), (E) myricetin (4).
