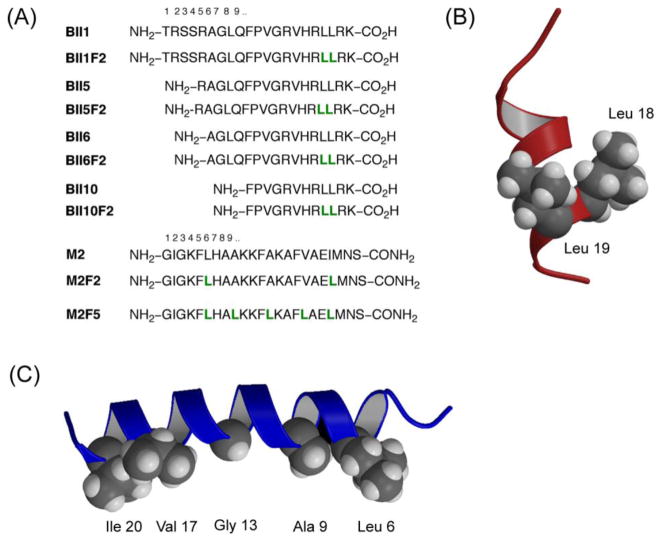
Figure 2.
Fluorinated antimicrobial peptides – analogues of buforin II (BII) and magainin 2 amide (M2) show increased proteolytic stability, and in six out of seven cases retention or enhanced activity. The highly fluorinated peptide M2F5 forms a helical bundle in water and is inactive against bacterial strains. (A) Peptide sequences in one letter code. Underlined residues were chosen for replacement (L = hexafluoroleucine). (b) Model structure of buforin indicating the location of residues that were replaced by hexafluoroleucine. (c) NMR structure of magainin 2 amide indicating the hydrophobic face where substitutions with fluorinated residues were made (PDB code: 2mag).