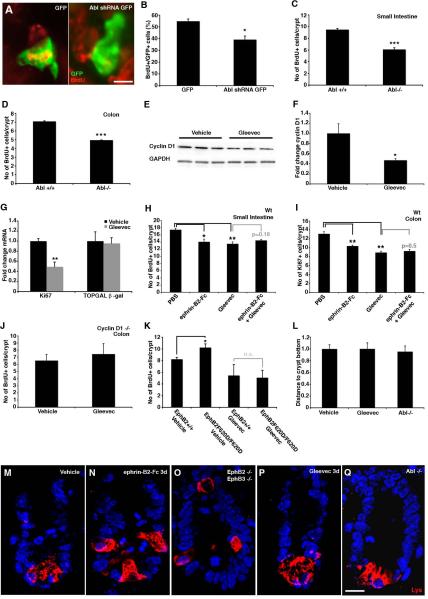
Figure 6. Proliferation, but not migration, is mediated by Abl.
(A and B) Electroporation of Abl shRNA in intestinal explants leads to a reduction in the number of electroporated cells (GFP expressing) incorporating BrdU. (C and D) Proliferation in the Abl mutant mouse is reduced in both small intestine and colon. (E and F) Gleevec (100mg/kg) leads to a decrease in cyclin D1 protein levels in the colon detected by Western blot analysis.
(G) Administration of Gleevec to TOPGAL mice leads to a 50% reduction in Ki67 transcript, without affecting the expression of β-galactosidase (p=0.87).
(H and I) Inhibiting Abl with Gleevec or ephrin-B2-Fc reduces cell proliferation in the small intestine and colon to a similar extent, but there is no additive effect.
(J) Gleevec does not affect cell proliferation in colon crypts in cyclin D1 −/− mice.
(K) Analysis of EphB2 F620D/F620D mice with constitutively active EphB2 receptors reveal an increase in the number of BrdU positive cells when compared to wild type littermates. Administration of Gleevec to wild type and EphB2 F620D/F620D mice results in a suppression of proliferation to the same level in both genotypes. (L–Q) Paneth cells, visualized by lysozyme (Lys)-immunoreactivity, are displaced in EphB2; EphB3 double null mutant animals. Inhibition of EphB signaling with ephrin-B2-Fc also results in displaced Paneth cells 3 days after an injection. In contrast, animals receiving Gleevec for three consecutive days as well as Abl −/− mice show no evidence of Paneth cell displacement.
n=3–6 mice in each group, except for n=2 cyclin D1 −/− mice receiving Gleevec. Data are represented as mean+SEM. * = p≤ 0.05, ** = p≤ 0.01, *** = p≤ 0.001, Student's t-test. Scale bar = 10 μm.