Abstract
Mono-ADP ribosylation of actin by bacterial toxins, such as Clostridium perfringens iota or Clostridium botulinum C2 toxins, results in rapid depolymerization of actin filaments and cell rounding. Here we report that treatment of African green monkey kidney (Vero) cells with iota toxin resulted in delayed caspase-dependent death. Unmodified actin did not reappear in toxin-treated cells, and enzyme-active toxin was detectable in the cytosol for at least 24 h. C2 toxin showed comparable, long-lived effects in cells, while a C2 toxin control lacking ADP-ribosyltransferase activity did not induce cell death. To address whether the remarkable stability of the iota and C2 toxins in cytosol was crucial for inducing cell death, we treated cells with C/SpvB, the catalytic domain of Salmonella enterica SpvB. Although C/SpvB also mono-ADP ribosylates actin as do the iota and C2 toxins, cells treated with a cell-permeating C/SpvB fusion toxin became rounded but recovered and remained viable. Moreover, unmodified actin reappeared in these cells, and ADP-ribosyltransferase activity due to C/SpvB was not detectable in the cytosol after 24 h, a result most likely due to degradation of C/SpvB. Repeated application of C/SpvB prevented recovery of cells and reappearance of unmodified actin. In conclusion, a complete but transient ADP ribosylation of actin was not sufficient to trigger apoptosis, implying that long-term stability of actin-ADP-ribosylating toxins, such as iota and C2, in the cytosol is crucial for inducing delayed, caspase-dependent cell death.
Various bacterial toxins destroy the actin cytoskeleton of eukaryotic cells by mono-ADP ribosylation of G-actin at arginine-177 (1, 3, 23). ADP-ribosylated actin caps the barbed, fast-growing ends of actin filaments (F-actin), thus preventing further assembly of unmodified G-actin into filaments (32). Although ADP-ribosylated G-actin does not affect the pointed, slow-growing ends of F-actin, the critical concentration for actin polymerization does increase and leads to complete depolymerization of actin filaments (33). Therefore, treatment of cells with these toxins disrupts the actin cytoskeleton and causes rounding of adherent cells within hours.
Binary ADP-ribosylating toxins that target actin can be divided into family members that include Clostridium botulinum C2 toxin (20), Clostridium perfringens iota toxin (29), CDT from Clostridium difficile (26), Clostridium spiroforme toxin, also known as CST (25), and the vegetative insecticidal proteins from Bacillus cereus (9). The clostridial and bacillus binary toxins are typical exotoxins, produced by extracellular bacteria, that ultimately enter the cytosol of targeted cells without the toxin-producing bacteria. In contrast, SpvB from Salmonella enterica (21, 31) also targets actin but is delivered into the host cell's cytosol by intracellularly located bacteria.
All binary actin-ADP-ribosylating toxins are composed of two nonlinked proteins, a binding/translocation component and a separate enzyme component (3). In recent years, the structures, modes of action, and cellular uptake mechanisms of the C2 and iota toxins have been discovered to various degrees. The binding/translocation components of the C2 (C2IIa) and iota (Ib) toxins, respectively, mediate cell surface docking of the enzyme components C2I and Ia, followed by cellular uptake and translocation of an enzyme component(s) from acidified endosomes into the cytosol (3). Although the C2 and iota toxins share comparable structures and modes of action, there are striking differences regarding modification of actin isoforms and individual steps during toxin internalization (3). Iota toxin, CDT, and CST are very closely related, and their components are interchangeable, unlike the C2 toxin (8, 22, 24, 25). Thus, CDT and CST are referred to as the iota-like toxins, with C. perfringens iota toxin representing the prototype. Iota toxin is an enterotoxin that naturally causes diarrhea in calves and lambs, is lethal for mice, and is dermonecrotic for guinea pigs (28, 29, 30). The iota-like toxins of C. difficile and C. spiroforme are also associated with gastrointestinal diseases of humans and animals (for a review, see reference 3).
Although the immediate cytopathic effects induced by iota toxin have been investigated in detail, the long-term effects on mammalian cells following intoxication and in particular the fate of internalized Ia ADP-ribosyltransferase are unknown. In this study, we have shown that iota toxin-mediated ADP ribosylation of actin and subsequent cell rounding are irreversible, resulting in delayed caspase-dependent death. We detected enzyme-active Ia in the cytosol 48 h after application of iota toxin to cells, indicating that the Ia domain harboring ADP-ribosyltransferase activity remained stable in the cytosol. Prompted by our recent observation that C2 toxin delays apoptosis and persists as an active ADP-ribosyltransferase in the cytosol of intoxicated cells (10), we have now addressed whether the long-lived nature of clostridial actin-ADP-ribosylating toxins in the cytosol is crucial for delayed cell death. To this end, we also investigated whether a fusion toxin containing the catalytic domain of S. enterica SpvB (C/SpvB) mono-ADP ribosylates G-actin as do the C2 and iota toxins (11, 27).
MATERIALS AND METHODS
Cell culture and media.
African green monkey kidney (Vero; ATCC CCL-81) cells were cultivated at 37°C in 5% CO2 and minimal essential medium (Invitrogen, Karlsruhe, Germany) containing 10% heat-inactivated fetal bovine serum, 17 mM sodium bicarbonate, 1 mM sodium pyruvate, 2 mM l-glutamine, and 0.1 mM nonessential amino acids. Cells were trypsinized and reseeded for a maximum of 20 cycles. To inhibit protein biosynthesis, Vero cells were incubated in the presence of 20 μM cycloheximide (CHX) (Roche, Mannheim, Germany).
Cytotoxicity assay.
Cells were seeded on culture dishes, grown to subconfluency in complete medium, and then incubated with toxin. Pictures of the cells were taken after the indicated incubation periods using an Axiovert 40CFl microscope from Zeiss (Oberkochen, Germany) connected to a Progress C10 charge-coupled-device camera from Jenoptik (Jena, Germany). Cell viability was determined by using the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] cytotoxicity assay Cell Titer 96 Aqueous from Promega (Mannheim, Germany) according to the manufacturer's instructions.
Expression and purification of recombinant proteins.
The C2I and C2II components of C. botulinum C2 toxin were expressed as glutathione-S-transferase (GST) fusion proteins in Escherichia coli BL21 (4). Recombinant proteins were purified by affinity chromatography and then incubated with thrombin (3.25 NIH units/ml of bead suspension) to cleave the GST domain. The GST domain was removed by incubation with benzamidine Sepharose. C2II was activated with trypsin for 30 min at 37°C as described previously (4). The C2IN-C/SpvB fusion protein was expressed as a recombinant GST construct in E. coli BL21 and purified as described earlier (27). Ib, Ia, and IaE378A were purified as described earlier (22). All purified toxin components were subjected to 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), yielding a single band following Coomassie blue staining, and protein concentrations were determined via densitometry by using the Photoshop 7.0 software program. Bovine serum albumin was used as a standard for all protein determinations.
Preparation of cell lysates and immunoblot analysis.
Following incubation with toxin, cells were washed twice with ice-cold phosphate-buffered saline (PBS) and then lysed in 20 mM Tris-HCl (pH 7.5) containing 1 mM EDTA, 1 mM dithiothreitol (DTT), 5 mM MgCl2, and Complete protease inhibitor (Roche, Mannheim, Germany). Following sonication and centrifugation (14,000 rpm, 6 min, 4°C) with a refrigerating microcentrifuge (Eppendorf 5417R; Eppendorf, Hamburg, Germany), the supernatant was stored at −20°C. For immunoblot analysis, equal amounts of lysate protein were subjected to SDS-PAGE (12.5% gels) according to the method of Laemmli (13). Subsequently, separated proteins were electrophoretically transferred to a nitrocellulose membrane (Macherey-Nagel, Dueren, Germany) and blocked for 30 min with 5% nonfat dry milk in PBS containing 0.05% Tween 20 (PBS-T). After incubation with the first antibody against specific proteins, membranes were washed with PBS-T and subsequently incubated for 1 h with the respective antispecies antibodies coupled to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were finally washed, and proteins were visualized using an enhanced chemiluminescence system (Millipore, Schwalbach, Germany) according to the manufacturer's instructions. For the detection of actin, samples were probed with a monoclonal anti-β-actin antibody (clone AC-15; Sigma, Deisenhofen, Germany). Detection of PARP-1 was performed using the mouse monoclonal antibody C-II-10 (14), kindly provided by A. Bürkle, Konstanz, Germany. Cleaved caspase-3 was detected with rabbit polyclonal antibody (Cell Signaling Technology, Danvers, MA).
ADP ribosylation assay.
For in vitro ADP ribosylation of actin, 20 to 50 μg of whole-cell lysate protein was incubated for 30 min at 37°C in a buffer containing 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM DTT, 5 mM MgCl2, and Complete protease inhibitor with C2I (300 ng) and 10 μM biotinylated NAD+ (R&D Systems, Minneapolis, MN). The reaction was stopped by adding 5× SDS-sample buffer (625 mM Tris-HCl [pH 6.8], 20% SDS, 8.5% glycerol, 0.2% bromophenol blue, 100 mM DTT) and heating at 95°C for 5 min. Samples were subjected to SDS-PAGE, and ADP-ribosylated actin was subsequently detected with peroxidase-coupled streptavidin (Roche, Mannheim, Germany) via enhanced chemiluminescence. Alternatively, in vitro ADP ribosylation of actin was performed with 32P-NAD+ (Hartmann Analytics, Braunschweig, Germany) as described earlier (4), and radioactively labeled proteins were detected by phosphorimaging.
Detection of Ia and C2I ADP-ribosyltransferases in the cytosol.
Vero cells were incubated with 0.2 μg Ia/ml plus 0.4 μg Ib/ml for the indicated times. Toxin was removed, and cells were further incubated at 37°C without toxin. At the given times, cells were washed with ice-cold PBS and then scraped from the plate in 20 mM Tris-HCl (pH 7.5) containing 1 mM EDTA, 1 mM DTT, and 5 mM MgCl2 supplemented with Complete protease inhibitor. Cells were lysed by transferring them 10 times through a 1-ml syringe with a 20-gauge needle. Subsequently, the cell preparation was centrifuged (100,000 × g, 1 h, 4°C) and the supernatant (cytosolic fraction) used to detect Ia or C2I activity. Alternatively, the cytosolic fractions were obtained by digitonin extraction as described earlier (10, 12). In brief, Vero cells were grown in 12-well plates and incubated with 0.2 μg Ia/ml plus 0.4 μg Ib/ml for the indicated times. Toxin was removed, and cells were further incubated at 37°C without toxin. At the given times, cells were washed with ice-cold PBS and the cytosolic proteins were extracted by a 5-min incubation at 25°C with 20 μg/ml digitonin (Sigma-Aldrich, Seelze, Germany) in PBS containing Complete protease inhibitor to permeabilize the cytoplasmic membrane. These cells were then incubated for 30 min at 4°C in this same buffer to facilitate leakage of cytosolic proteins. The cytosolic fraction was used for detection of enzyme-active Ia by in vitro ADP ribosylation. Cytosolic fractions were incubated for 1 h at 37°C with fresh cell lysate as a source of actin and biotinylated NAD+ as a cosubstrate (R&D Systems, Wiesbaden, Germany). Alternatively, a bioassay with naive Vero cells was performed as described recently (10). Cytosolic samples from cells incubated with iota or C2 toxin were applied to naive cells in combination with fresh Ib or C2IIa, respectively, for the indicated times at 37°C, and the percentage of rounded cells was determined after various incubation periods.
To detect Ia or C2I in the cytosolic fractions, Vero cells were grown in 12-well plates and incubated for 40 min at 4°C in serum-free medium with biotin-labeled Ia (0.4 μg/ml) plus Ib (0.8 μg/ml) or with biotin-labeled C2I (0.4 μg/ml) plus C2IIa (0.8 μg/ml) to enable toxin binding. Medium was subsequently removed, and cells were washed with ice-cold PBS followed by incubation at 37°C in toxin-free medium for the indicated times. The cytosolic fraction was obtained by digitonin extraction as described before, and aliquots (25 μg of protein each) were subjected to SDS-PAGE. Separated proteins were transferred to a nitrocellulose membrane probed with streptavidin-peroxidase for the presence of biotinylated Ia or C2I.
Microinjection of C/SpvB and C2I.
Vero cells were seeded in 3-cm dishes on cover slides with grids, and C/SpvB or C2I (100 μM in microinjection buffer) or microinjection buffer alone (20 mM Tris-HCl, pH 7.5) was injected into the cells with an Eppendorf 5242 microinjector. After the indicated incubation periods, pictures from identical cells were taken.
Reproducibility of experiments and statistics.
All experiments were performed independently at least twice. Results from representative experiments are shown in the figures. Values (n ≥ 3) are calculated as means ± standard deviations (SD) using the GraphPad Prism4 software program, and statistical significance was determined by using Student's t test.
RESULTS
ADP ribosylation of actin and changes in cell morphology induced by iota toxin are irreversible and elicit caspase-dependent death.
We initially investigated the long-term responses of Vero cells after treatment with iota toxin. Vero cells incubated for 3 h with Ia (0.2 μg/ml) plus Ib (0.4 μg/ml) became uniformly round, the toxin-containing medium was removed, and cells were further incubated in medium without toxin. In parallel, the toxin was not removed from other wells, and these cells were incubated for 24 h with iota toxin. As a control, cells were incubated in medium without iota toxin. For the cells incubated for 24 h with iota toxin and cells incubated with iota toxin for 3 h followed by fresh medium minus toxin, there was no recovery, as evidenced by uniform rounding after 24 h (Fig. 1A). Recovery of intoxicated cells was not observed after longer incubation periods; however, cells that detached from the substratum became more numerous. As additional controls, the individual Ia or Ib component had no obvious effect upon cell morphology after 24 h (data not shown).
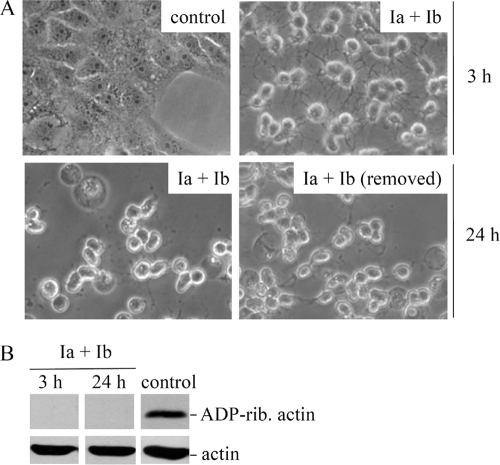
FIG. 1.
Morphology and ADP ribosylation status of actin from iota toxin-treated Vero cells. (A) Vero cells were incubated for 3 h at 37°C with iota toxin (0.2 μg Ia/ml plus 0.4 μg Ib/ml) or medium alone as a control. After 3 h, toxin-treated cells were subsequently incubated at 37°C with either fresh medium alone (Ia plus Ib removed) or continued toxin treatment up to 24 h. Pictures were taken at given times to document morphological changes. (B) Cells were incubated for 3 h or 24 h with iota toxin (0.2 μg Ia/ml plus 0.4 μg Ib/ml) or left untreated (medium only) as a control. The ADP ribosylation status of actin from these cells was analyzed by in vitro ADP ribosylation of cell lysates with biotin-labeled NAD+. Biotin-labeled, ADP-ribosylated actin is shown in the upper panel, while the bottom panel reveals common amounts of total actin detected by specific antibody.
We next analyzed the ADP ribosylation status of actin from cells treated with iota toxin for 3 h or 24 h (Fig. 1B, upper panel). The results clearly indicated that all actin was ADP ribosylated by toxin within intact cells after just 3 h, and unmodified actin did not reappear in the cells by 24 h, even in fresh medium. The total amount of cellular actin was not influenced by toxin, thus indicating that ADP ribosylation by iota toxin did not accelerate G-actin degradation (Fig. 1B, lower panel). In conclusion, the cytopathic effects of iota toxin (ADP ribosylation of actin and cell rounding) were not transient or reversible. We next investigated whether iota toxin treatment results in cell death.
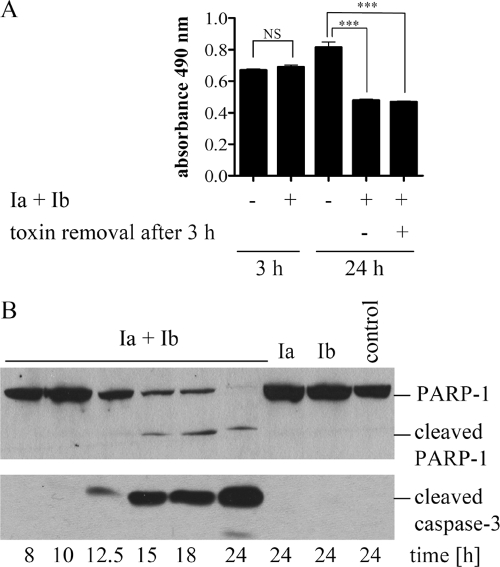
Cells were treated with iota toxin exactly as described above, and viability was measured with the MTS assay after 3 and 24 h. While toxin-treated cells were still viable after 3 h, a significant decrease in viability was detected after 24 h (Fig. 2A). Decreased viability was independent of whether toxin was removed from the medium after 3 h or remained for an additional 21 h. Since conclusions regarding the type of cell death cannot be made from the MTS assay, we performed specific experiments to test whether iota toxin-induced death was apoptotic. We first analyzed the time-dependent cleavage of PARP-1, a substrate for caspase-3, in iota toxin-treated Vero cells as a specific feature of apoptosis. Immunoblot results revealed that the PARP-1 cleavage fragment (85 kDa) first appeared 15 h after toxin application (Fig. 2B, upper panel). Cleavage of PARP-1 was detectable only when Ia plus Ib were applied to cells but not in the absence of toxin or presence of individual components. PARP-1 is a substrate for caspase-3; therefore, we analyzed cleavage of procaspase-3 to an activated form in the same cells with a specific antibody against cleaved caspase-3 (Fig. 2B, lower panel). Caspase-3 activation first appeared 12.5 h following application of iota toxin and then increased upon further incubation of the cells with toxin. As expected, cleaved caspase-3 was not detectable in untreated cells or with individual toxin components. Thus, iota toxin treatment of Vero cells resulted in activation of caspase-3 ∼12.5 h later and consequent cleavage of the caspase-3 substrate, PARP-1. Altogether, these results clearly indicated that iota toxin induces delayed apoptosis.
FIG. 2.
Iota toxin-induced delayed death of Vero cells correlates with PARP-1 cleavage and caspase-3 activation. (A) Measurement of cell viability by MTS assay. Vero cells were incubated at 37°C for 3 h or 24 h with iota toxin (0.2 μg Ia/ml plus 0.4 μg Ib/ml) or left untreated as a control. Alternatively, cells were treated for 3 h with iota toxin, and the toxin-containing medium was replaced with fresh medium for an additional 21 h. After 3 h or 24 h, cells were incubated for an additional 1 h at 37°C with the MTS reagent, and the resulting formazan was measured at 490 nm. Values are given as means ± SD (n = 3 determinations in the same experiment). Statistical significance was determined between control cells and cells treated with iota toxin by the Student t test (***, P < 0.0005; NS, not significant). (B) Detection of cleaved PARP-1 and caspase-3 as two hallmarks of apoptosis. Vero cells were incubated for the indicated time points at 37°C with iota toxin (0.2 μg Ia/ml plus 0.4 μg Ib/ml), individual toxin components, or medium alone (control). Cells were lysed, and proteins were subjected to immunoblot analysis. Cleavage of the caspase substrate PARP-1 was monitored with C-II-10 antibody (upper panel). In parallel, the proteolytic activation of procaspase-3 was analyzed with a specific antibody against cleaved caspase-3 (lower panel).
ADP ribosylation of actin is essential to induce delayed apoptosis.
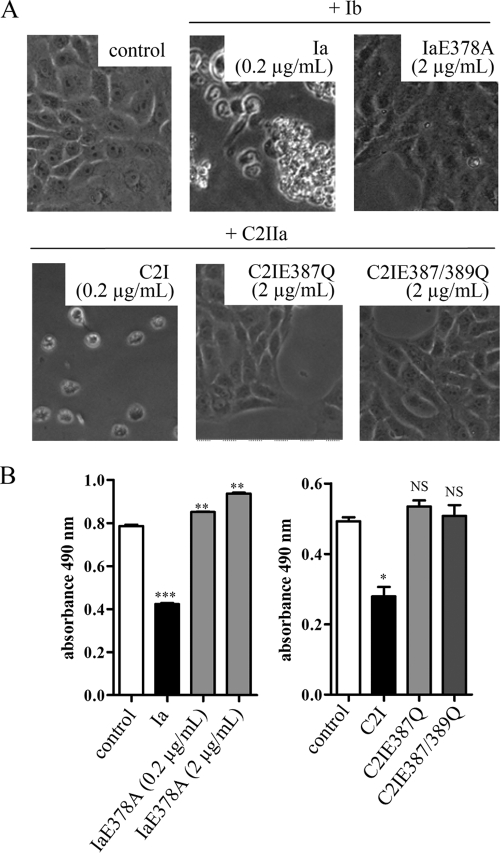
Prompted by these findings, we next tested whether cell death induced by actin-specific, ADP-ribosylating toxins depends exclusively on ADP-ribosyltransferase activity. To this end, we investigated the long-term responses of Vero cells after treatment with IaE378A, a variant of Ia lacking ADP-ribosyltransferase activity (22). Together with E380, E378 is part of the biglutamic acid motif in the catalytic site of Ia and represents an essential residue for ADP-ribosyltransferase, but not NAD-glycohydrolase, activity (22). After 48 h, cells treated with Ia plus Ib were round and most cells detached from the substratum (Fig. 3A, upper panel). In contrast, IaE378A plus Ib did not change the morphology of Vero cells, even when both proteins were applied in a 10-fold-higher concentration (2 μg/ml IaE378A plus 4 μg/ml Ib) to the cells (Fig. 3A). In combination with Ib, only wild-type Ia but not IaE378A induced cell death, as demonstrated by an MTS cytotoxicity assay (Fig. 3B).
FIG. 3.
ADP-ribosyltransferase-deficient IaE378A, C2IE387Q, and C2IE387/389Q do not induce cell death. Vero cells were treated at 37°C either with wild-type Ia (0.2 μg/ml) in combination with Ib (0.4 μg/ml) or with IaE378A (0.2 μg/ml or 2 μg/ml) in combination with Ib (0.4 μg/ml or 4 μg/ml, respectively). In parallel, Vero cells were incubated with C2IIa (0.4 μg/ml) and wild-type C2I (0.2 μg/ml) or C2IIa (4 μg/ml) in combination with either C2IE387Q (2 μg/ml) or C2IE387/389Q (2 μg/ml). While C2IE387 is devoid of ADP-ribosyltransferase activity but retains full NAD-glycohydrolase activity, C2IE387/389Q lacks both activities. Control cells were incubated in medium without toxin (control). After 48 h, pictures were taken to document changes in cell morphology (A) and viability was measured by an MTS assay (B). Values in panel B are means ± SD (n = 3 determinations in the same experiment). Statistical significance was determined between control cells and those treated with either wild-type or mutated C2 toxins using the Student t test (***, P < 0.0005; **, P < 0.005; *, P < 0.03; NS, not significant).
We confirmed this observation by using C2 toxin as another actin-ADP-ribosylating toxin and investigated the long-term effects of two different C2I variants, C2IE387Q and C2IE387/389Q, which represent the biglutamic acid motif in C2I and both of which lack ADP-ribosyltransferase activity (5). Importantly, C2IE387Q but not C2IE387/389Q still retains full NAD-glycohydrolase activity (5). After 48 h with C2IIa plus wild-type C2I, Vero cells became rounded. In contrast, C2IIa combined with either C2IE387Q or C2IE387/389Q had no influence on cell morphology even when 10-fold-higher concentrations of C2IE387Q and C2IE387/389Q, versus wild-type C2I, were applied to cells (Fig. 3A, lower panel). Importantly, we confirmed in parallel that C2IE387Q was internalized into the cytosol by C2IIa, comparable to results with wild-type C2I (data not shown). In contrast to wild-type C2I, C2IE387Q and C2IE387/389Q did not trigger cell death within 48 h (Fig. 3B). In line with these results, the ADP-ribosyltransferase-deficient C2I proteins did not induce cleavage of PARP-1 (data not shown). Overall, these results clearly indicated that ADP-ribosyltransferase, but not NAD-glycohydrolase, activity of actin ADP-ribosylating toxins induced cell death.
Ia retains ADP-ribosyltransferase activity in the cytosol for at least 48 h.
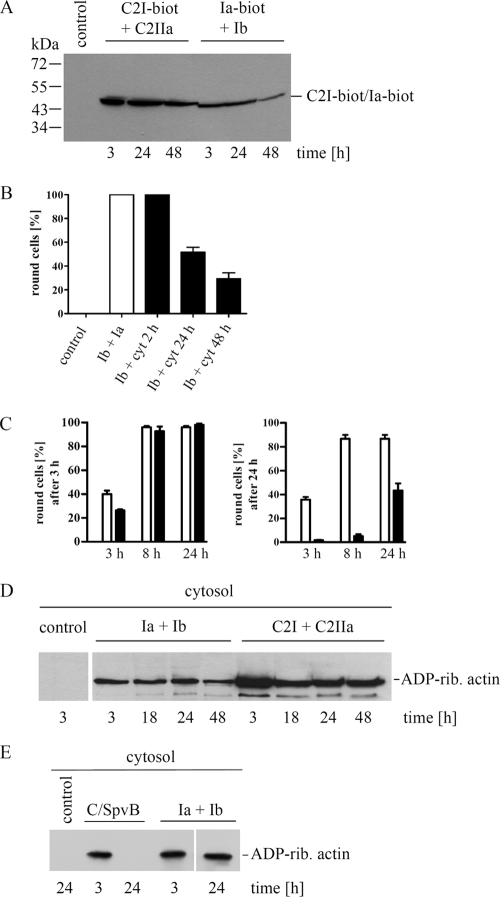
We monitored the fate of internalized Ia in the cytosol of intoxicated Vero cells using biotin-labeled Ia in direct comparison to results with internalized biotin-labeled C2I. Initial experiments confirmed that biotin labeling did not influence cellular uptake or enzyme activity of Ia or C2I. Vero cells were incubated with either Ib (0.8 μg/ml) plus biotinylated Ia (0.4 μg/ml) or C2IIa (0.8 μg/ml) plus biotinylated C2I (0.4 μg/ml) for 3 h to allow internalization of Ia or C2I, while control cells were incubated in medium only. After 3 h, the toxin-treated cells were uniformly round, the medium was removed, and cells were further incubated in toxin-free medium. Cytosolic fractions of the cells were obtained by digitonin extraction either immediately after toxin removal or following additional incubation periods of 24 h and 48 h without toxin. The cytosolic fractions were analyzed for biotinylated Ia or biotinylated C2I with streptavidin-peroxidase (Fig. 4A). There was no signal in control cells incubated with medium alone; however, a comparable amount of biotin-labeled C2I was detectable after 3, 24, and 48 h in the cytosolic fractions of C2 toxin-treated Vero cells. In the cytosolic fractions of iota toxin-treated Vero cells, there was no detectable decrease in the amount of biotin-labeled Ia within 24 h, while the amount of Ia decreased significantly between 24 and 48 h. Biotinylated Ia and biotinylated C2I, which were run as controls on the same gel, migrated to the same positions on the gel (∼45 kDa; data not shown) and thus confirmed that the detected proteins were indeed Ia and C2I. This finding indicates that although there was some loss of Ia between 24 and 48 h of incubation, most likely due to degradation in the cytosol, residual Ia remained in the cytosol many hours after the initial intoxication. However, we did not detect Ia degradation products in those blots.
FIG. 4.
Detection of Ia and ADP-ribosyltransferase activity in the cytosol of intoxicated Vero cells. (A) Vero cells were incubated in 12-well plates for 3 h at 37°C with Ib (0.8 μg/ml) plus biotin-labeled Ia (Ia-biot) (0.4 μg/ml) or alternatively with C2IIa (0.8 μg/ml) plus biotin-labeled C2I (C21-biot) (0.4 μg/ml). The toxin-containing medium was removed, and cells were further incubated at 37°C. The cytosolic fractions were obtained by digitonin extraction for Western blot detection of biotinylated Ia or biotinylated C2I with streptavidin-peroxidase. (B) Determination of biologically active Ia from the cytosol of intoxicated cells. Cells were incubated for 2 h with Ia (0.2 μg/ml) plus Ib (0.4 μg/ml) or left untreated with medium alone (control). The toxin was removed from the medium, and cells were further incubated for 24 and 48 h. Cells were lysed, and the cytosolic fractions (cyt) were obtained by ultracentrifugation. Aliquots from the cytosolic fractions were applied to naive Vero cells with fresh Ib in medium. These cells were incubated at 37°C, and after 24 h the percentage of rounded cells was determined as an indicator of Ia activity. As a positive control, fresh Ia plus fresh Ib were applied to the cells (indicated as Ia + Ib; white bar). Values are given as means ± SD (n = 3 determinations from the same experiment). (C) Comparison of biologically active Ia and C2I from the cytosol of intoxicated Vero cells. Cells in 12-well plates were incubated at 37°C for 3 h with either Ia (0.2 μg/ml) plus Ib (0.4 μg/ml) or C2I (0.2 μg/ml) plus C2IIa (0.4 μg/ml). The toxins were removed from the medium, and cells were incubated for an additional 8 and 24 h. The cytosolic fractions of iota- or C2-intoxicated cells were obtained by digitonin extraction, combined with either fresh Ib (black bars) or fresh C2IIa (white bars), respectively, and then applied to naive Vero cells to determine biological activities of cytoslic Ia or C2I. After 3 and 24 h, the percentage of round cells was determined. Values are given as means ± SD (n = 3 determinations from the same experiment). (D) Time course for ADP-ribosyltransferase activity of Ia and C2I from cytosolic fractions of iota- or C2-intoxicated cells. Vero cells were incubated at 37°C for 3 h in 12-well plates with Ia (0.2 μg/ml) plus Ib (0.4 mg/ml), C2I (0.2 μg/ml) plus C2IIa (0.4 μg/ml), or medium alone as a control. Toxin-containing medium was removed, and cells were further incubated without toxin for the indicated times. Cells were then washed, and the cytosolic fractions were obtained by digitonin treatment. To measure the ADP-ribosyltransferase activities of Ia and C2I in cytosolic fractions, aliquots were incubated for 30 min at 37°C with biotin-NAD+ and fresh cell lysate used as an actin source. The biotin-labeled (i.e., ADP-ribosylated [ADP-rib.]) actin is shown. (E) Another mono-ADP-ribosylating toxin that targets actin, C2IN-C/SpvB, was tested in comparison to iota toxin in this assay. Vero cells were incubated with either C2IIa (1 μg/ml) plus C2IN-C/SpvB (2 μg/ml) or Ia (0.2 μg/ml) plus Ib (0.4 μg/ml) for 3 h. The toxins were removed, and cells were either lysed or further incubated in toxin-free medium for 21 h before lysis. Control cells were incubated with medium alone. All additional experimental steps were performed exactly as described above.
We next investigated whether cytosolic Ia was still active up to 48 h after intoxication by combining fresh Ib with cytosolic Ia and then adding this mixture to naive Vero cells. Vero cells were incubated for 2 h with Ia plus Ib, toxin was removed from the medium, and cells were incubated for an additional 24 h or 48 h in medium only. The cytosolic fractions were prepared, and aliquots from the cytosolic fractions were applied to Vero cells with fresh Ib. Cell rounding was monitored after 24 h to detect whether Ia from the cytosol of previously intoxicated cells remained biologically active. As a negative control, the cytosolic fraction from untreated cells was applied with Ib to cells. A positive control included application of fresh Ia plus Ib to cells. Figure 4B shows percentages of toxin-induced rounding among intoxicated cells compared to medium-only controls after 24 h. Approximately 50% of cells were round when fresh Ib was applied with the cytosolic fraction from cells previously treated with iota toxin for 24 h. Approximately 30% of cells were round after treatment with cytosols from iota-intoxicated cells incubated for 48 h. These results demonstrate that internalized (cytosolic) Ia remains biologically active (i.e., docks with Ib and causes rounding of naive cells) even when Ia has been in the cytosol for 48 h. This finding was consistent with our Western blot detection of Ia up to 48 h after internalization into the cytosol.
However, when the biological activities of Ia and C2I from cytosolic fractions of iota or C2 toxin-treated Vero cells were directly compared in the same assay, there was a significant difference. While C2I did not lose much of its biological activity between 3 and 24 h in the cytosol, activity of cytosolic Ia dramatically decreased from 3 h to 24 h (Fig. 4C).
Finally, we performed a time course analysis of the ADP-ribosyltransferase activity of internalized Ia in direct comparison to the activity of internalized C2I. To this end, Vero cells were incubated with either Ia (0.2 μg/ml) plus Ib (0.4 μg/ml) or C2I (0.2 μg/ml) plus CIIa (0.4 μg/ml) for 3 h until cells were uniformly round, toxins were removed from the medium, and cells were then incubated in medium up to 48 h. Aliquots from the cytosolic fractions were analyzed for ADP-ribosyltransferase activity by incubation with biotin-NAD+ and fresh Vero cytosol as a source of actin. As shown in Fig. 4D, there were no major differences in the amount of ADP-ribosylated actin modified by the cytosolic fractions from cells treated with iota toxin for 3, 18, 24, or 48 h. A comparable result was obtained for C2 toxin (Fig. 4D).
Taken together, the results clearly indicated that even after 48 h there was still very robust, enzymatically active Ia or C2I in the cytosol, suggesting minimal degradation of the catalytic C-terminal domain of Ia or C2I (Fig. 4D). In slight contrast, there was a time-dependent decrement in biological activity of cytosolic Ia when combined with that of fresh Ib and naive cells (Fig. 4B). Such findings could hint at cytosolic degradation or improper folding of the N-terminal domain of Ia, which mediates docking of Ia to Ib on the cell surface.
In contrast to Ia, long-lived ADP-ribosyltransferase activity in the cytosol was not evident for the catalytic domain (C/SpvB) of SpvB from Salmonella enterica, which like the C2 and iota toxins also mono-ADP-ribosylates actin at arginine-177. We treated Vero cells with the C2IN-C/SpvB fusion plus C2IIa (protein transporter of C2IN-C/SpvB into the cytosol) for 3 h to allow internalization into the cytosol. Subsequently, toxin was removed from the medium and cells were incubated in medium alone for an additional 21 h. The cytosolic fractions were obtained by digitonin treatment and analyzed for ADP-ribosyltransferase activity, exactly as described above for iota toxin. There was ADP-ribosyltransferase activity in the cytosol from cells treated for 3 h with C2IIa plus C2IN-C/SpvB but not in the cytosol after further incubation (21 h) in medium only (Fig. 4E). This is in contrast to results for Ia in the same experiment, thus suggesting that C/SpvB is less stable in the cytosol than Ia. One reason for this observation might be that C2IN-C/SpvB is a recombinant domain fusion protein that is perhaps more susceptible to degradation than the natural C2 and iota toxins.
Transient mono-ADP ribosylation of actin does not induce delayed apoptosis.
We next addressed whether long-term stability of ADP-ribosyltransferase activity in the cytosol is crucial for toxin-induced, delayed cell death. If so, the C2IN-C/SpvB fusion toxin, which mono-ADP ribosylates actin but does not persist as an active ADP-ribosyltransferase in the host cell cytosol as long as Ia or C2I, should not induce cell death (11, 27). We first investigated the long-term effects of C/SpvB regarding morphological alterations and ADP ribosylation of actin. Vero cells were incubated for 3 h with C2IN-C/SpvB plus C2IIa until all cells were round (Fig. 5A). When those cells were washed and incubated for an additional 21 h in medium only, most recovered and regained a flat, pleomorphic morphology (Fig. 5A). Time-based recovery was not restricted to Vero cells, since this effect was also observed for HeLa, Ptk-2, CHO, CaCo-2, and J774.A1 cells (data not shown).
FIG. 5.
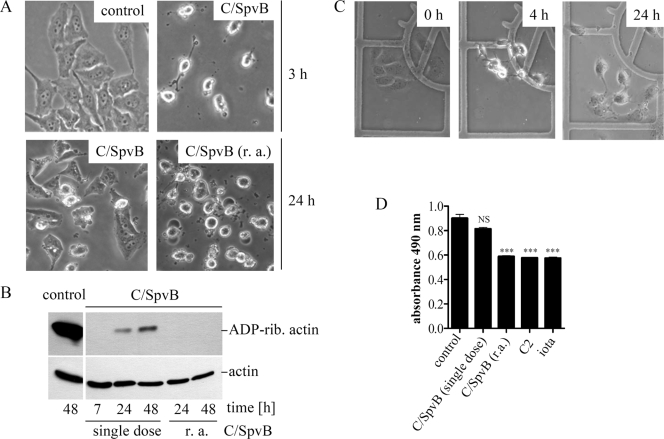
Recovery of C/SpvB-treated Vero cells. (A) Morphology of Vero cells after treatment with C2IIa plus C2IN-C/SpvB (C/SpvB). C2IN-C/SpvB (2 μg/ml) and C2IIa (1 μg/ml) were applied to cells in a single dose, followed by incubation at 37°C for the indicated times. In one sample, designated r. a. (repeated application), fresh C2IIa and C2IN-C/SpvB proteins were initially applied to the cells and then applied every 7 h thereafter. Control cells were incubated with medium only. Pictures were taken after 3 h and 24 h to monitor changes in cell morphology. (B) ADP ribosylation status of actin from Vero cells after treatment with C2IIa plus C2IN-C/SpvB. Vero cells were treated as described for panel A. At the indicated times, cells were lysed and lysate proteins (10 μg total) subjected to in vitro ADP ribosylation of actin. Relative actin amounts (bottom panel) were determined by a specific antibody. (C) Recovery of Vero cells after microinjection of C/SpvB. C/SpvB (100 μM in injection buffer) was microinjected into Vero cells grown on grids in six-well plates (indicated as 0 h; left panel) and at 4 h after microinjection of C/SpvB (middle panel, showing rounding). Cells were incubated for an additional 20 h, and morphological changes were monitored. (D) Viability of Vero cells after 24 h with various actin-targeting, ADP-ribosylating toxins. Cells were incubated with the following toxins: C2 (0.2 μg/ml C2I plus 0.4 μg/ml C2IIa), iota (0.2 μg/ml Ia plus 0.4 μg/ml Ib), or a single dose of C/SpvB (2 μg/ml C2IN-C/SpvB plus 1 μg/ml C2IIa). Alternatively, C2IN-C/SpvB and C2IIa were applied every 7 h. After 24 h, cell viability was determined with the MTS assay and absorbance of triplicate wells was measured at 490 nm. Values are given as the means ± SD (n = 3 determinations from the same experiment). Statistical significance was determined between control and toxin-treated cells by using Student's t test (***, P < 0.0005; NS, not significant).
Based on our finding that enzyme activity of C/SpvB was lost in the cytosol of Vero cells within 24 h, we next tested whether loss of enzyme activity in the cytosol could be overcome by repeated application of fresh toxin every 7 h. Indeed, recovery of rounded cells was not observed when fresh C2IIa and C2IN/CSpvB were applied repeatedly (Fig. 5A). When the ADP ribosylation status of actin from such cells was analyzed, all cellular actin was ADP ribosylated after a 7-h treatment with toxin. Biotin-labeled actin was detected after in vitro ADP ribosylation with biotin-NAD+ in lysates from cells treated for 24 h or 48 h with a single dose of C2IIa plus C2IN-C/SpvB (Fig. 5B, upper panel). This result clearly indicates that such lysates contained unmodified actin that reappeared by 24 h in cells after a single application of C2IIa plus C2IN-C/SpvB. In contrast, no biotin-labeled actin was detected in cells treated every 7 h with the toxin (Fig. 5B, upper panel, indicated as r. a. for repeated application), implying that there was no unmodified actin in the lysates of those cells which could serve as a substrate during the subsequent in vitro ADP ribosylation with biotin-NAD+. The reappearance of unmodified actin correlated with a recovery of cell morphology, thus implying that the loss of C/SpvB ADP-ribosyltransferase activity in the cytosol led to newly synthesized actin not being ADP ribosylated and to reconstitution of F-actin structures. When comparable amounts of either C/SpvB or C2I (100 μM) were microinjected into Vero cells, there was uniform rounding after 4 h. However, when the identical cells were monitored for morphological changes over time, the C/SpvB-treated cells regained a flat shape after 24 h (Fig. 5C). In contrast, C2I-treated cells did not recover, and instead, all C2I-treated cells were round but detached from the substratum after 24 h (data not shown).
We tested whether treatment of Vero cells with C2IN-C/SpvB in combination with C2IIa resulted in delayed cell death. Vero cells treated with a single dose of C2IIa/C2IN-C/SpvB remained viable for several days, while repeated application of C2IIa/C2IN-C/SpvB induced cell death within 24 h, just as with iota or C2 toxin treatment (Fig. 5D).
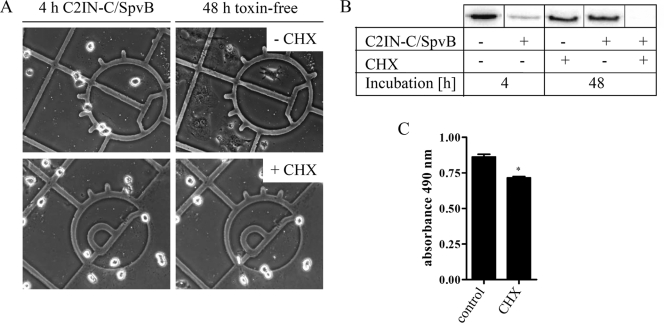
Finally, we investigated whether the reappearance of unmodified actin in cells after recovery from C/SpvB was due to de novo synthesis of actin. To prove this point, Vero cells were incubated for 4 h with C2IN-C/SpvB plus C2IIa until all cells were round (Fig. 6A). Subsequently, the cells were incubated in toxin-free medium for an additional 48 h with or without CHX to prevent protein biosynthesis. Recovery of cells was prevented in the presence of CHX (Fig. 6A). When the ADP ribosylation status of actin from the cells was analyzed by in vitro ADP ribosylation, it became evident that CHX prevented the reappearance of unmodified actin (Fig. 6B). It is noteworthy that we confirmed by MTS cytotoxicity assay that the majority of Vero cells were still viable after 48 h of cycloheximide treatment (Fig. 6C). These results imply that de novo synthesis of actin was essential for recovery of C2IN-C/SpvB-treated cells.
FIG. 6.
Recovery of C/SpvB-treated Vero cells and reappearance of unmodified actin are prevented by CHX. (A) Vero cells were incubated for 4 h at 37°C with C2IN-C/SpvB (1 μg/ml) plus C2IIa (1 μg/ml). Subsequently, the toxin was removed from the medium and cells were further incubated in the absence or presence of 20 μM CHX up to 48 h after initial toxin treatment. (B) Cells were treated exactly as described above, and after 48 h, the cells were lysed and the ADP ribosylation status of actin was determined by a radioactive in vitro ADP ribosylation assay. The 32P-labeled, ADP-ribosylated actin is shown. (C) Viability of Vero cells after 48 h of treatment with CHX was determined using the MTS assay, and absorbance of triplicate wells was measured at 490 nm. Values are given as the means ± SD (n = 3 determinations from the same experiment). Statistical significance was determined between control and CHX-treated cells by using Student's t test.
Taken together, the results clearly indicate that a complete mono-ADP ribosylation of actin by C2IN-C/SpvB is not sufficient to induce delayed, caspase-dependent cell death like that caused by either the iota or C2 toxin. When the ADP-ribosyltransferase activity of C2IN-C/SpvB was lost in the cytosol over time, newly synthesized actin was not ADP ribosylated, and this was most likely the underlying reason for the observed recovery of intoxicated cells.
DISCUSSION
Prompted by our recent observation that C2 toxin from C. botulinum triggers delayed, caspase-dependent death of epithelial cells (10), we have now performed a series of experiments to address the long-term responses of Vero cells after treatment with C. perfringens iota toxin. Both of these clostridial toxins are composed of binary, ADP-ribosylating proteins that specifically target G-actin. Although iota toxin induced 100% cell rounding within 3 h, intoxicated cells remained curiously viable 15 h after toxin application. Despite some structural and enzymatic differences between the C2 and iota-like toxins, they are quite comparable regarding their nonreversible cytopathic mode of action and time course of toxin-mediated cell death. Iota toxin treatment of Vero cells caused caspase-3 activation within 12 to 15 h after application, which favorably compares to the time course of caspase-3 activation in C2 toxin-treated HeLa cells (10).
Further investigation revealed the long-lived nature of enzyme-active Ia in the cytosol as being very comparable to what we observed earlier for C2I (10). There was no obvious decrease in ADP-ribosyltransferase activity of cytosolic Ia or C2I within 24 h; however, there was an interesting difference between Ia and C2I obtained from cytosolic fractions concerning biological activity. The cytosolic fraction from cells treated for 24 h with iota toxin, when applied to naive cells with fresh Ib, yielded 50% cell rounding after 20 h. In contrast, ∼50% of the cells were round after just 3 h when the cytosolic fraction of C2 toxin-treated cells was incubated with naive cells and fresh C2IIa (10). The relatively low biological activity of cytosolic Ia is likely due to reduced docking with fresh Ib on the cell surface and/or impaired translocation of Ia by Ib from acidified endosomes into the cytosol.
For both C2I and Ia, the active site containing highly conserved, catalytic residues is located within the C-terminal domain (5). Because our results indicate long-term stability of the Ia catalytic domain, there was most likely degradation and/or misfolding of that Ia region (residues 129 to 257), importantly involved in Ib interactions on the cell surface and/or translocation of Ia into the cytosol (16). Between 24 and 48 h, there were decreased amounts of biotinylated Ia in the host cell cytosol, but there was only a slight decrease of the Ia ADP-ribosyltransferase activity in those same cytosols. This finding suggests that the C-terminal catalytic domain of Ia was stable in the cytosol even after 48 h, while the N-terminal portion of Ia might become degraded over time. However, we were not able to detect by Western blotting any degradation products of biotin-Ia in the cytosol with streptavidin-peroxidase. One explanation might be that biotin labeling of Ia occurred in the N-terminal region of the protein, which became degraded, and thus, the Ia catalytic domain alone might not be recognized by streptavidin-peroxidase. In contrast, the N-terminal domain of C2I, which mediates interaction with C2IIa and translocation of C2I, was still functional after 24 h in the cytosol (10). Differences between Ia and C2I are plausible after earlier observations that removal of 207 N-terminal residues from Ia yields a molecule that still ADP ribosylates actin (8). Removal of just 29 N-terminal amino acids from C2I inactivates ADP-ribosyltransferase activity (8). Residues 129 to 257 of Ia, which mediate interaction with Ib and subsequent translocation, are not required for enzymatic activity; therefore, degradation of this region within Ia after translocation into the cytosol would not impair ADP-ribosyltransferase activity in the cytosol. We postulate that cytosolic stability of ADP-ribosylating toxins targeting actin is a prerequisite for triggering delayed cell death.
Ia and C2I, which were deficient in ADP-ribosyltransferase activity (5, 22), did not trigger cell death. However, ADP-ribosylating toxins like C2 and iota also harbor NAD-glycohydrolase activity (18, 19); therefore, we tested whether that activity also led to toxin-mediated cell death. By using recombinant C2I that lacks ADP-ribosyltransferase activity but retains full NAD-glycohydrolase activity (5), we excluded such a hypothesis and demonstrated that only ADP ribosylation of actin is essential for toxin-mediated cell death.
A hypothesis that the long-lived nature of ADP-ribosyltransferase activity in the cytosol of intoxicated cells is necessary for toxin-mediated death is further supported by results with another ADP-ribosyltransferase, C/SpvB. Our findings indicated that complete yet transient ADP ribosylation of actin by C/SpvB mediated the same immediate cytopathic effects (i.e., rounding) but not death. Notably, we used the C/SpvB fusion protein as a tool to unravel the impact of actin-ADP ribosylation upon apoptosis (27). Therefore, unequivocal conclusions cannot be drawn from these experiments concerning the mode of action for full-length SpvB after translocation into the cytosol of cells containing Salmonella.
According to our current model, the long-lived nature of clostridial ADP-ribosyltransferases in the cytosol leads to ADP ribosylation of any newly synthesized G-actin, thus preventing critical reconstitution of actin filaments/cytoskeleton and recovery of intoxicated cells. Permanent ADP ribosylation of actin by persistent, enzymatically active ADP-ribosyltransferase domains is most likely essential for the toxin-mediated, delayed cell death which we have now discovered for the actin-specific, binary ADP-ribosylating toxins from various Clostridium species.
In addition to the iota and C2 toxins, initial experiments from our laboratory have also revealed that Vero cells incubated with another binary ADP-ribosylating toxin, CDT, synthesized by particularly virulent strains of C. difficile, undergo delayed cell death (unpublished observation). These preliminary results further strengthen our belief that the mechanism described in detail above for the iota and C2 toxins might be common for all actin-specific, binary ADP-ribosylating toxins from clostridia. Moreover, this observation could have an impact on further understanding of the role of CDT in C. difficile-associated pathogenesis. A hypervirulent and epidemic strain of C. difficile, which shows increased resistance to fluoroquinolones, produces increased levels of toxins A and B, as well as the binary toxin CDT. There is evidence from epidemiological studies that production of CDT by C. difficile is associated with more-severe diarrhea, implying that strains harboring CDT are more virulent than those not expressing the binary toxin (2, 6, 7, 15, 17).
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) Priority Program SPP 1150 grant to H.B. (no. BA 2087/1-3).
We thank Ulrike Binder (Ulm) for excellent technical assistance. We thank Alexander Bürkle (Konstanz) for kindly providing the anti-PARP-1 antibody C-II-10.
Editor: A. Camilli
Footnotes
Published ahead of print on 5 October 2009.
REFERENCES
- 1.Aktories, K., M. Bärmann, I. Ohishi, S. Tsuyama, K. H. Jakobs, and E. Habermann. 1986. Botulinum C2 toxin ADP-ribosylates actin. Nature 322:390-392. [DOI] [PubMed] [Google Scholar]
- 2.Barbut, F., B. Gariazzo, L. Bonne, V. Lalande, B. Burghoffer, R. Luiuz, and J. C. Petit. 2007. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000-2004. Infect. Control Hosp. Epidemiol. 28:131-139. [DOI] [PubMed] [Google Scholar]
- 3.Barth, H., K. Aktories, M. R. Popoff, and B. G. Stiles. 2004. Binary bacterial toxins: biochemistry, biology, and applications of common Clostridium and Bacillus proteins. Microbiol. Mol. Biol. Rev. 68:373-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth, H., D. Blöcker, J. Behlke, W. Bergsma-Schutter, A. Brisson, R. Benz, and K. Aktories. 2000. Cellular uptake of Clostridium botulinum C2 toxin requires oligomerization and acidification. J. Biol. Chem. 275:18704-18711. [DOI] [PubMed] [Google Scholar]
- 5.Barth, H., J. C. Preiss, F. Hofmann, and K. Aktories. 1998. Characterization of the catalytic site of the ADP-ribosyltransferase Clostridium botulinum C2 toxin by site-directed mutagenesis. J. Biol. Chem. 273:29506-29511. [DOI] [PubMed] [Google Scholar]
- 6.Blossom, D. B., and L. C. McDonald. 2007. The challenges posed by reemerging Clostridium difficile infection. Clin. Infect. Dis. 45:222-227. [DOI] [PubMed] [Google Scholar]
- 7.Geric, B., S. Johnson, D. N. Gerding, M. Grabnar, and M. Rupnik. 2003. Frequency of binary toxin genes among Clostridium difficile strains that do not produce large clostridial toxins. J. Clin. Microbiol. 41:5227-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulke, I., G. Pfeifer, J. Liese, M. Fritz, F. Hofmann, K. Aktories, and H. Barth. 2001. Characterization of the enzymatic component of the ADP-ribosyltransferase toxin CDTa from Clostridium difficile. Infect. Immun. 69:6004-6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han, S., J. A. Craig, C. D. Putnam, N. B. Carozzi, and J. A. Tainer. 1999. Evolution and mechanism from structures of an ADP-ribosylating toxin and NAD complex. Nat. Struct. Biol. 6:932-936. [DOI] [PubMed] [Google Scholar]
- 10.Heine, K., S. Pust, S. Enzenmuller, and H. Barth. 2008. ADP-ribosylation of actin by the Clostridium botulinum C2 toxin in mammalian cells results in delayed caspase-dependent apoptotic cell death. Infect. Immun. 76:4600-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochmann, H., S. Pust, G. von Figura, K. Aktories, and H. Barth. 2006. Salmonella enterica SpvB ADP-ribosylates actin at position arginine-177—characterization of the catalytic domain within the SpvB protein and a comparison to binary clostridial actin-ADP-ribosylating toxins. Biochemistry 45:1271-1277. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser, E., S. Pust, C. Kroll, and H. Barth. 2009. Cyclophilin A facilitates translocation of the Clostridium botulinum C2 toxin across membranes of acidified endosomes into the cytosol of mammalian cells. Cell Microbiol. 11:780-795. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.Lamarre, D., B. Talbot, G. De Murcia, C. Laplante, Y. Leduc, A. Mazen, and G. G. Poirier. 1988. Structural and functional analysis of poly(ADP ribose) polymerase: an immunological study. Biochim. Biophys. Acta 950:147-160. [DOI] [PubMed] [Google Scholar]
- 15.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 16.Marvaud, J. C., B. G. Stiles, A. Chenal, D. Gillet, M. Gibert, L. A. Smith, and M. R. Popoff. 2002. Clostridium perfringens iota toxin. Mapping of the Ia domain involved in docking with Ib and cellular internalization. J. Biol. Chem. 277:43659-43666. [DOI] [PubMed] [Google Scholar]
- 17.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 18.Nagahama, M., Y. Sakaguchi, K. Kobayashi, S. Ochi, and J. Sakurai. 2000. Characterization of the enzymatic component of Clostridium perfringens iota-toxin. J. Bacteriol. 182:2096-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohishi, I. 1986. NAD-glycohydrolase activity of botulinum C2 toxin: a possible role of component I in the mode of action of the toxin. J. Biochem. 100:407-413. [DOI] [PubMed] [Google Scholar]
- 20.Ohishi, I., M. Iwasaki, and G. Sakaguchi. 1980. Purification and characterization of two components of botulinum C2 toxin. Infect. Immun. 30:668-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto, H., D. Tezcan-Merdol, R. Girisch, F. Haag, M. Rhen, and F. Koch-Nolte. 2000. The spvB gene-product of the Salmonella enterica virulence plasmid is a mono(ADP-ribosyl)transferase. Mol. Microbiol. 37:1106-1115. [DOI] [PubMed] [Google Scholar]
- 22.Perelle, S., M. Domenighini, and M. R. Popoff. 1996. Evidence that Arg-295, Glu-378, and Glu380 are active-site residues of the ADP-ribosyltransferase activity of iota toxin. FEBS Lett. 395:191-194. [DOI] [PubMed] [Google Scholar]
- 23.Perelle, S., M. Gibert, P. Bourlioux, G. Corthier, and M. R. Popoff. 1997. Production of a complete binary toxin (actin-specific ADP-ribosyltransferase) by Clostridium difficile CD196. Infect. Immun. 65:1402-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perelle, S., S. Scalzo, S. Kochi, M. Mock, and M. R. Popoff. 1997. Immunological and functional comparison between Clostridium perfringens iota toxin, C. spiroforme toxin, and anthrax toxins. FEMS Microbiol. Lett. 146:117-121. [DOI] [PubMed] [Google Scholar]
- 25.Popoff, M. R., and P. Boquet. 1988. Clostridium spiroforme toxin is a binary toxin which ADP-ribosylates cellular actin. Biochem. Biophys. Res. Commun. 152:1361-1368. [DOI] [PubMed] [Google Scholar]
- 26.Popoff, M. R., E. J. Rubin, D. M. Gill, and P. Boquet. 1988. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 56:2299-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pust, S., H. Hochmann, E. Kaiser, G. von Figura, K. Heine, K. Aktories, and H. Barth. 2007. A cell-permeable fusion toxin as a tool to study the consequences of actin-ADP-ribosylation caused by the Salmonella enterica virulence factor SpvB in intact cells. J. Biol. Chem. 282:10272-10282. [DOI] [PubMed] [Google Scholar]
- 28.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stiles, B. G., and T. D. Wilkins. 1986. Purification and characterization of Clostridium perfringens iota toxin: dependence on two nonlinked proteins for biological activity. Infect. Immun. 54:683-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiles, B. G., and T. D. Wilkins. 1986. Clostridium perfringens iota toxin: synergism between two proteins. Toxicon 24:767-773. [DOI] [PubMed] [Google Scholar]
- 31.Tezcan-Merdol, D., T. Nyman, U. Lindberg, F. Haag, F. Koch-Nolte, and M. Rhen. 2001. Actin is ADP-ribosylated by the Salmonella enterica virulence-associated protein SpvB. Mol. Microbiol. 39:606-619. [DOI] [PubMed] [Google Scholar]
- 32.Wegner, A., and K. Aktories. 1988. ADP-ribosylated actin caps the barbed ends of actin filaments. J. Biol. Chem. 263:13739-13742. [PubMed] [Google Scholar]
- 33.Weigt, C., I. Just, A. Wegner, and K. Aktories. 1989. Nonmuscle actin ADP-ribosylated by botulinum C2 toxin caps actin filaments. FEBS Lett. 246:181-184. [DOI] [PubMed] [Google Scholar]