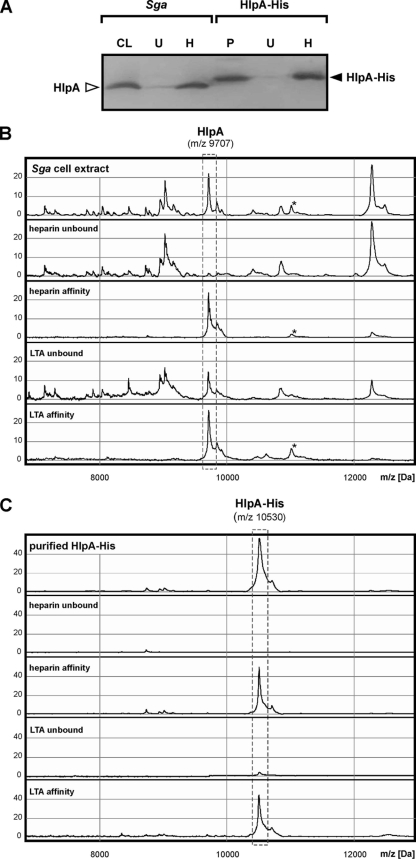
FIG. 4.
Heparin and LTA affinity of HlpA. (A) Western blot analysis of a total S. gallolyticus (Sga) cell lysate (CL) and purified (P) HlpA-His and the respective fractions that remain unbound (U) after incubation with immobilized heparin and that have heparin affinity (H). Blots were decorated with anti-HlpA or anti-six-His antibodies. The positions of endogenous HlpA and recombinant HlpA-His are indicated. (B) Low-molecular-mass protein profiles of a total S. gallolyticus cell extract, the respective proteins that remain unbound after incubation with immobilized heparin or LTA, and the respective proteins that have heparin or LTA affinity. Note that a second (unknown) protein with both heparin and LTA affinity is indicated with an asterisk. (C) Low-molecular-mass spectra of purified HlpA-His from S. gallolyticus, the fraction that remains unbound after incubation with immobilized heparin or LTA, and the proteins that have heparin or LTA affinity. The 9,707- and 10,530-Da peaks, corresponding to, respectively, endogenous HlpA and recombinant HlpA-His, are indicated. The peak intensity is given in arbitrary units.