Abstract
In a deep, subalpine holo-oligomictic lake, the relative abundance of Archaea and Crenarchaeota, but not that of Bacteria, increases significantly with depth and varies seasonally. Cell-specific prokaryotic productivity is homogeneous along the water column. The concept of active Archaea observed in the deep ocean can therefore be extended to a deep oxic lake.
The abundance, activity, and community composition of epilimnetic and hypolimnetic prokaryotes have been less thoroughly investigated in deep lakes than in oceans. Strong evidence that the depth gradient plays a role in modulating the balance between the domains of Bacteria and Archaea has been found in various marine systems (8, 12, 13, 20). It has been shown that the percentage of Bacteria in the deep marine hypolimnion decreases (up to 5,000 m) while, conversely, the percentage of Archaea increases. The percentage of Crenarchaeota is also higher in the mesopelagic zone than in surface waters (10).
Although Archaea have been found in a variety of freshwater habitats (3), little has thus far been published on differentiating between Bacteria, Archaea, and Crenarchaeota in the hypolimnion of deep lakes. An exception is a study of the high-altitude ultraoligotrophic Crater Lake (21, 22), where group I marine Crenarchaeota were observed in deep-water populations (22). This study and another study of various lakes from three continents (9) are based on summer sampling, making it impossible to ascertain the effects of temporal variability on the vertical distribution of Archaea and Crenarchaeota, as has been done for marine systems and shallow lakes (for examples, see references 8 and 11).
Our primary objective was to follow variations in the relative abundance of Bacteria, Archaea, and Crenarchaeota found in the hypolimnetic waters of a deep holo-oligomictic lake with a permanent oxic hypolimnion and compare them with those in the epilimnetic assemblages. We used the catalyzed reporter deposition fluorescence in situ hybridization (CARD-FISH) technique and compared the data thus obtained with prokaryotic productivity.
Environmental characteristics and samplings.
Lake Maggiore is a large, deep, subalpine lake (surface, 212 km2; maximum depth, 372 m) in Northern Italy included in the LTER network (http://www.ise.cnr.it/lter). The lake has recovered from an eutrophic state (15) and is now oligomesotrophic (2, 14). A particular hydrodynamic feature of Lake Maggiore is that the full winter overturn occurs only at the end of a particularly cold and windy period (1).
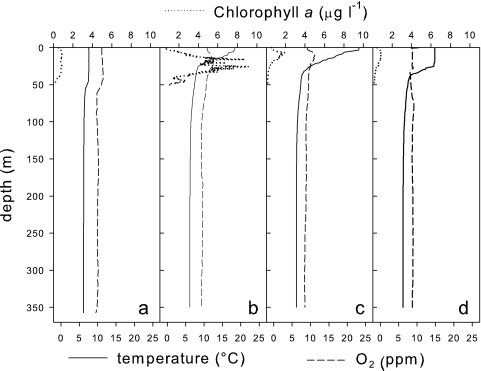
Temperature profiles (IDRONAUT OS316 multiparameter probe) indicated that stratification began in June (Fig. 1). The oxygen profiles showed that the water column was in a uniform oxic condition. Samples were taken in February, June, August, and October 2007 at 3 m, 10 m, 200 m, and 350 m, immediately fixed, and stored at −4°C (1 month).
FIG. 1.
Profiles of temperature, dissolved oxygen (O2), and in situ chlorophyll a in samples taken from Lake Maggiore on 14 February (a), 5 June (b), 7 August (c), and 23 October (d) 2007.
Prokaryote community composition.
CARD-FISH analyses for Archaea, Bacteria, and Crenarchaeota were performed in two replicates according to Pernthaler et al. (17) and Teira et al. (19). We used the following oligonucleotide probes (Thermo-Hybrid, Germany): Archaea, Arch915; Bacteria, Eub338, Eub338-II, and Eub338-III (5); and Crenarchaeota, Cren537. The hybridization for Bacteria ranged from 49% to 71% of total DAPI (4′,6-diamidino-2-phenylindole) counts and was comparable to published data on aquatic systems (7, 16). The difference between the Bacteria/Archaea ratio in the hypolimnion and that in the epilimnion highlights the increasing relative contribution of Archaea to total DAPI counts in deep water layers. During late summer stratification, Archaea account for 47% of total counts at 350 m (Fig. 2). When all data were considered, the relative abundance of Archaea was significantly higher in the hypolimnion (P = 0.028 by the Mann-Whitney U test) than in the epilimnion. Bacteria generally accounted for more than 50% of total DAPI counts. There was no significant difference in their relative abundances between the epilimnion and hypolimnion (P = 0.505 by the Mann-Whitney U test). Crenarchaeota accounted for a significantly higher percentage in the hypolimnion (P < 0.001 by the Mann-Whitney U test), following the same pattern as Archaea. They contributed from 30% (mean in epilimnion) to 70% (mean in hypolimnion) of the total archaeal counts.
FIG. 2.
Profiles of bacterioplankton composition from CARD-FISH with rRNA-targeted oligonucleotide probes for Bacteria (Eub338, EubII, and EubIII), Archaea (Arch915), and Crenarchaeota (Cren537). The histograms represent means ± standard errors. Samples were taken from Lake Maggiore on 14 February (a), 5 June (b), 7 August (c), and 23 October (d) 2007.
Our results agree with those obtained in oceans (4, 8, 20), where pelagic Archaea and Crenarchaeota were found to increase at greater depths. Our data from a deep subalpine lake add new insight into the vertical distribution of Bacteria, Archaea, and Crenarchaeota under various limnological conditions during the year. There is a close relationship between marine Crenarchaeota and their counterparts in large lakes, based on identical lipid structures (3). This evidence suggests that in the deep hypolimnion of lakes, the pelagic Crenarchaeota may act as chemoautotrophs, oxidizing ammonia to nitrate (6) and fixing inorganic carbon in the dark (23). The cold hypolimnion of Lake Maggiore is permanently oxygenated and may provide a niche for uncultivated mesophilic Crenarchaeota.
Depth profiles of prokaryotic abundance and productivity.
Total DAPI counts were used to calculate the abundance of Bacteria, Archaea, and Crenarchaeota from the percentage of hybridized cells. The average cell numbers of Bacteria, Archaea, and Crenarchaeota in the epilimnion and hypolimnion were 25.6 × 105 to 5.4 × 105 cells ml−1, 7.4 × 105 to 2.5 × 105 cells ml−1, and 1.5 × 105 to 1.6 × 105 cells ml−1, respectively (Fig. 3). Abundances of Bacteria and Archaea were significantly different in the epilimnion and hypolimnion (P < 0.001 [Bacteria] and P = 0.028 [Archaea] by the Mann-Whitney U test), while no difference was observed in the abundances of Crenarchaeota (P = 0.645 by the Mann-Whitney U test).
FIG. 3.
Depth profiles of absolute abundance (cell ml−1) of Bacteria, Archaea, and Crenarchaeota. The bars represent standard errors. Samples were taken from Lake Maggiore on 14 February (filled inverted triangles), 5 June (open triangles), 7 August (filled circles), and 23 October (open squares) 2007.
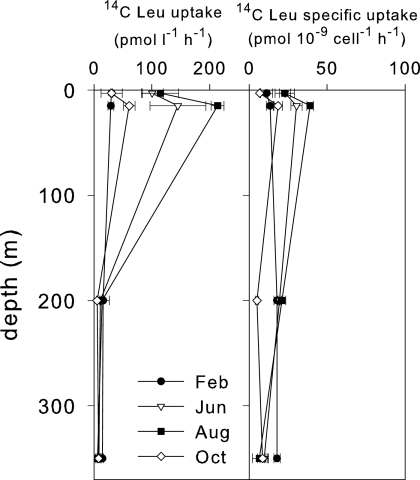
Prokaryotic productivity was measured by [14C]leucine ([14C]Leu) according to Smith and Azam (18), with minor modifications. [14C]Leu uptake scaled to cell number showed a vertical homogeneous distribution in February, while in August there was a peak in the epilimnion (39 pmol [14C]Leu 10−9 cell−1 h−1) (Fig. 4). No significant difference was observed between epilimnetic and hypolimnetic cell-specific [14C]Leu uptake (P = 0.113 by analysis of variance). Conversely, total production levels were significantly different in the two zones (P < 0.001 by analysis of variance) (Fig. 4).
FIG. 4.
Profiles of total productivity ([14C]Leu uptake) and cell-specific productivity ([14C]Leu-specific uptake) of prokaryotes. The bars represent standard errors. Samples were taken from Lake Maggiore on 14 February (filled circles), 5 June (open inverted triangles), 7 August (filled squares), and 23 October (open diamonds) 2007.
The cell-specific productivity of prokaryotes did not change with depth. This evidence, together with the increasing relative abundance of Archaea and Crenarchaeota in the hypolimnion, supports the hypothesis that, in a deep lake, Archaea may be an active component of the prokaryotic community, as they have been found to be in oceans (20). Also, similar temporal fluctuations in archaeal abundances suggest that Archaea have an effective role in microbial communities, as has been observed in oceans (4).
Conclusions.
Our results from in situ measurements demonstrated relatively unchanged cell-specific productivity along the water column, indicating that prokaryotes living in the hypolimnion are on average as active as those living in the epilimnion. The observed increment with depth in the relative abundance of Archaea and Crenarchaeota was always significant, although it varied unevenly through the seasons. The different abundances of Bacteria and Archaea in the epilimnion and hypolimnion suggest that they contribute differently to overall microbial activity in the two zones.
Acknowledgments
We thank Stefano Amalfitano (CNR-IRSA, Rome, Italy) for his support in executing the CARD-FISH protocol. We also thank our anonymous reviewers for their useful comments on the manuscript.
Footnotes
Published ahead of print on 18 September 2009.
REFERENCES
- 1.Ambrosetti, W., L. Barbanti, and N. Sala. 2003. Residence time and physical processes in lakes. J. Limnol. 62(Suppl.):1-15. [Google Scholar]
- 2.Bertoni, R., R. Piscia, and C. Callieri. 2004. Horizontal heterogeneity of seston, organic carbon and picoplankton in the photic zone of Lago Maggiore, Northern Italy. J. Limnol. 63:244-249. [Google Scholar]
- 3.Casamayor, E. O., and C. Borrego. 2009. Archaea, p. 167-181. In G. E. Likens (ed.), Encyclopedia of inland waters, vol. 3. Elsevier, Oxford, United Kingdom. [Google Scholar]
- 4.Church, M. J., E. F. DeLong, H. W. Ducklow, M. B. Karner, C. M. Preston, and D. M. Karl. 2003. Abundance and distribution of planktonic Archaea and Bacteria in the waters west of the Antarctic Peninsula. Limnol. Oceanogr. 48:1893-1902. [Google Scholar]
- 5.Daims, H., A. Bruhl, R. Amann, K. H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 6.Francis, C. A., J. M. Beman, and M. M. M. Kuypers. 2007. New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. ISME J. 1:19-27. [DOI] [PubMed] [Google Scholar]
- 7.Glöckner, F. O., B. M. Fuchs, and R. Amann. 1999. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl. Environ. Microbiol. 65:3721-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karner, M. B., E. F. DeLong, and D. M. Karl. 2001. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507-510. [DOI] [PubMed] [Google Scholar]
- 9.Keough, B. P., T. M. Schmidt, and R. E. Hicks. 2003. Archeal nucleic acids in picoplankton from great lakes on three continents. Microb. Ecol. 46:238-248. [DOI] [PubMed] [Google Scholar]
- 10.Kirchman, D. L., H. Elifantz, A. I. Dittel, R. R. Malmstrom, and M. T. Cottrell. 2007. Standing stocks and activity of Archaea and Bacteria in the western Arctic Ocean. Limnol. Oceanogr. 52:495-507. [Google Scholar]
- 11.Lliros, M., E. O. Casamayor, and C. Borrego. 2008. High archaeal richness in the water column of a freshwater sulfurous karstic lake along an interannual study. FEMS Microbiol. Ecol. 66:331-342. [DOI] [PubMed] [Google Scholar]
- 12.Massana, R., A. E. Murray, C. M. Preston, and E. F. DeLong. 1997. Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara Channel. Appl. Environ. Microbiol. 63:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massana, R., L. J. Taylor, A. E. Murray, K. Y. Wu, W. H. Jeffrey, and E. F. DeLong. 1998. Vertical distribution and temporal variation of marine planktonic Archaea in the Gerlache Strait, Antarctica, during early spring. Limnol. Oceanogr. 43:607-617. [Google Scholar]
- 14.Morabito, G., D. Ruggiu, and P. Panzani. 2002. Recent dynamics (1995-1999) of the phytoplankton assemblages in Lago Maggiore as a basic tool for defining association patterns in the Italian deep lakes. J. Limnol. 61:129-145. [Google Scholar]
- 15.Mosello, R., and D. Ruggiu. 1985. Nutrient load, trophic condition and restoration prospects of Lake Maggiore. Int. Rev. Gesamten Hydrobiol. 70:63-75. [Google Scholar]
- 16.Pernthaler, A., J. Pernthaler, and R. Amann. 2002. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 68:3094-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pernthaler, A., J. Pernthaler, and R. Amann. 2004. Sensitive multicolor fluorescence in situ hybridization for the identification of environmental microrganisms, p. 711-726. In A. D. L. Akkermans, F. J. de Bruijn, and J. D. van Elsas (ed.), Molecular microbial ecology manual, 2nd ed. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 18.Smith, D. C., and F. Azam. 1992. A simple, economical method for measuring bacterial protein synthesis in seawater using 3H-leucine. Mar. Microb. Food Webs 6:107-114. [Google Scholar]
- 19.Teira, E., T. Reinthaler, A. Pernthaler, J. Pernthaler, and G. J. Herndl. 2004. Combining catalyzed reporter deposition-fluorescence in situ hybridization and microautoradiography to detect substrate utilization by bacteria and archaea in the deep ocean. Appl. Environ. Microbiol. 70:4411-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teira, E., H. van Aken, C. Veth, and G. J. Herndl. 2006. Archaeal uptake of enantiomeric amino acids in the meso- and bathypelagic waters of the North Atlantic. Limnol. Oceanogr. 51:60-69. [Google Scholar]
- 21.Urbach, E., K. L. Vergin, G. L. Larson, and S. J. Giovannoni. 2007. Bacterioplankton communities of Crater Lake, OR: dynamic changes with euphotic zone food web structure and stable deep water populations. Hydrobiologia 574:161-177. [Google Scholar]
- 22.Urbach, E., K. L. Vergin, L. Young, A. Morse, G. L. Larson, and S. J. Giovannoni. 2001. Unusual bacterioplankton community structure in ultra-oligotrophic Crater Lake. Limnol. Oceanogr. 46:557-572. [Google Scholar]
- 23.Wuchter, C., S. Schouten, H. T. S. Boschker, and J. S. S. Damste. 2003. Bicarbonate uptake by marine Crenarchaeota. FEMS Microbiol. Lett. 219:203-207. [DOI] [PubMed] [Google Scholar]