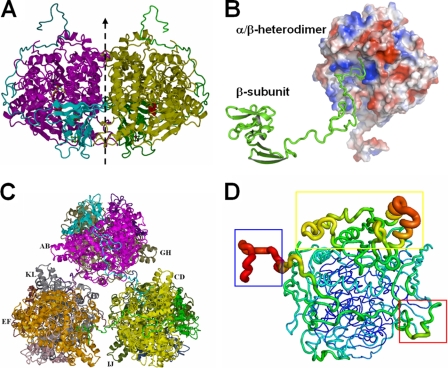
FIG. 3.
Structure of the APSR hexamer from D. gigas. (A) Structure of the α2β2-heterotetramer from D. gigas. The αβ-heterodimer performs a rotation around a twofold axis (dashed line) with another αβ-heterodimer to form a tightly contacted α2β2-heterotetramer. (B) Interactions between the αβ-heterodimers and the C terminus of another β-subunit. The long C-terminal loop of the β-subunit (shown in green ribbon) plugs into the active channel of another αβ-heterodimer (shown in electrostatic surface; the positive potential is in blue, and the negative potential is in red). (C) View of the hexamer structure. Three α2β2-heterotetramers contact each other through the C-termini of the β-subunits to form a hexamer containing six αβ-heterodimers. (D) B-factor-labeled structure of the αβ-heterodimer. The structure exhibits a high B-factor in red and the larger caliber of the cartoon style. The blue box shows the high B-factor at the C terminus (aa 144 to 167) of the β-subunit. The electron-accepting site on the β-subunit that is suggested to interact with an unknown electron donor shows a higher B-factor in the red box. The capping domain in the α-subunit also shows a higher B-factor in the yellow box.