Abstract
Hepatitis C virus (HCV) genotype 4 (HCV-4) infection is considered to be difficult to treat and has become increasingly prevalent in European countries, including The Netherlands. Using a molecular epidemiological approach, the present study investigates the genetic diversity and evolutionary origin of HCV-4 in Amsterdam, The Netherlands. Phylogenetic analysis of the NS5B sequences (668 bp) obtained from 133 patients newly diagnosed with HCV-4 infection over the period from 1999 to 2008 revealed eight distinct HCV-4 subtypes; the majority of HCV-4 isolates were of subtypes 4d (57%) and 4a (37%). Three distinct monophyletic clusters were identified, with each one having a specific epidemiological profile: (i) Egyptian immigrants infected with HCV-4a (n = 46), (ii) Dutch patients with a history of injecting drug use infected with HCV-4d (n = 44), and (iii) Dutch human immunodeficiency virus (HIV)-positive men who have sex with men (MSM) infected with HCV-4d (n = 26). Subsequent molecular clock analyses confirmed that the emergence of HCV-4 within these three risk groups coincided with (i) the parenteral antischistosomal therapy campaigns in Egypt (1920 to 1960), (ii) the popularity of injecting drug use in The Netherlands (1960 to 1990), and (iii) the rise in high-risk sexual behavior among MSM after the introduction of highly active antiretroviral therapy (1996 onwards). Our data show that in addition to the influx of HCV-4 strains from countries where HCV-4 is endemic, the local spread of HCV-4d affecting injecting drug users and, in recent years, especially HIV-positive MSM will further increase the relative proportion of HCV-4-infected patients in The Netherlands. HCV-4-specific agents are drastically needed to improve treatment response rates and decrease the future burden of HCV-4-related disease.
Hepatitis C virus (HCV) affects an estimated 170 million people worldwide. HCV infection persists in 50 to 85% of those infected and can, over decades, lead to cirrhosis and hepatocellular carcinoma (11). The HCV genome displays considerable sequence divergence, and HCV variants have been classified into seven major genotypes. Genotypes 1, 2, 3, 4, and 6 are further subdivided into numerous subtypes (subtypes a, b, c, etc.) (27). In the absence of complete genome sequences, the designation of a subtype is based mainly on consensus regions in the core/E1 and NS5B regions of the HCV genome (27). The HCV genotype distribution depends on the geographical region and the mode of transmission. As the distribution of HCV genotypes can change over time, genotyping provides a powerful tool that may be used to investigate the spread of HCV within a community (18).
In Europe, North America, and Australia, most HCV-infected patients (>80%) are infected with genotype 1, 2, or 3 (10). HCV genotype 4 (HCV-4) is the most common genotype in the Middle East and in northern and central Africa, accounting for more than 20% of all chronic HCV infections worldwide (28). In Egypt, the country with the highest prevalence of HCV in the world, more than 90% of patients are infected with HCV-4 (22). HCV-4 is considered difficult to treat and has a sustained virological response rate of approximately 60% (28), where the rates are 40 to 50% for genotype 1 and 80 to 90% for genotypes 2 and 3 (17).
Recent studies emphasize that the prevalence of HCV-4 in Europe has increased in the past few decades due to the immigration of HCV carriers and the subsequent spread of HCV-4 in European populations at risk for HCV infection (2, 5, 16, 24, 25, 30). In southern Europe, HCV-4 is responsible for 10 to 24% of chronic HCV infections. In The Netherlands, HCV-4 accounts for an estimated 10% of chronic HCV infections (6, 32). Currently, the development of new genotype-specific antiviral agents is focused mainly on HCV genotype 1. The emergence of HCV-4 may require agents specific for HCV-4 to improve the response rates and decrease the future burden of HCV-4 disease. The population of the region around Amsterdam, The Netherlands, comprises many ethnicities and diverse groups at risk for HCV infection, providing the opportunity to explore changes in the epidemiology of HCV-4. The aim of the study described here was to increase our understanding of the spread of HCV-4 in The Netherlands by using a molecular epidemiological approach. To our knowledge, this is the second study to have used phylogenetic analysis of HCV-4 isolates from a large cohort to investigate the genetic diversity of HCV-4 in Europe (14) and the first to include evolutionary analysis to describe the origin and spread of these subtypes.
MATERIALS AND METHODS
Study population.
The participants included all patients who were diagnosed with HCV-4 infection at the hepatology ward of the Academic Medical Center (AMC) from 1999 through 2008, as well as persons who were found to be HCV-4 positive at the Amsterdam Health Service (AHS). All AHS participants were part of surveys performed to monitor the general population or specific risk groups for the presence of sexually transmitted and blood-borne infections. AHS establishes the HCV genotype using an in-house PCR targeting the HCV core region (33). AMC uses a commercial 5′ untranslated region-based HCV genotyping assay (Trugene 5′NC; Genelibrarian module 3.1.2; Bayer Healthcare, Berkeley, CA). For this study, we collected data on the age, country of birth, human immunodeficiency virus (HIV) infection status, and plausible route of HCV transmission for each HCV-4-infected patient.
RNA isolation, reverse transcription, and PCR.
RNA was isolated from the first RNA-positive serum sample obtained from each patient by using 200 μl serum and a MagNa Pure total nucleic acid extraction kit (Roche Diagnostics, Almere, The Netherlands). HCV RNA was eluted in 100 μl of TE (Tris-EDTA) buffer and was subsequently transcribed to cDNA as described by Beld et al. (3). This cDNA was used as the input for two separate PCR assays targeting the HCV nonstructural 5B (NS5B) region, which resulted in the amplification of two overlapping NS5B fragments with a total length of 669 bp. Sera and RNA isolates were stored at −80°C.
NS5B PCR 1.
NS5B fragment 1 (338 bp, nucleotides 7938 to 8275) was amplified by a single-round PCR assay, as described by Murphy et al. (13). In brief, 12.5 μl of cDNA was added to a 12.5-μl reaction mixture containing 1× PCR ΙΙ buffer; 200 μmol/liter each of dATP, dCTP, and dGTP plus 400 μmol/liter dUTP; 0.1 μg/μl bovine serum albumin; 0.9 μM sense primer (5′-TATGAYACCCGCTGYTTTGACTC-3′); 0.9 μM antisense primer (5′-TAYCTVGTCATAGCCTCCGTGAA-3′); 0.5 U uracil N-glycosylase; and 2.5 U Amplitaq Gold (Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands). The final MgCl2 concentration was 2.5 mM. The PCR cycling conditions were 5 min at 95°C, followed by 45 cycles, with each cycle consisting of 30 s at 95°C, 30 s at 55°C, and 60 s at 72°C, plus a final period of incubation of 10 min at 72°C.
NS5B PCR 2.
NS5B fragment 2 (436 bp, nucleotides 8206 to 8642) was amplified by a nested PCR assay, as described by van de Laar et al. (33). The composition of the reaction mixture and the cycling conditions were identical to those described above, except some modifications were made so that 3 μl cDNA instead of 3 μl RNA could be used as the input for the first-round PCR. In the second-round PCR, we used only the reaction mixture containing HCV-4 genotype-specific primers 4HCV-IS (5′-CTGAGAGACTGCACSATGYTGGT-3′) and E1b (5′-AATGCGCTRAGRCCATGGAGTC-3′).
Sequencing and phylogenetic analysis.
The sequencing reactions were performed as described earlier (33). The viral genotype was confirmed by phylogenetic analysis of the NS5B sequences obtained, in which the sequences were compared with established reference sequences in the GenBank database (27). Phylogenetic trees were constructed by the maximum-likelihood approach implemented in PHYML software (9). Evolutionary model selection was performed after comparison of the corrected Akaike information criterion scores of various models by using PHYML software and the MrAIC script (15). The Hasegawa-Kishino-Yano substitution model with a γ distribution of among-site-rate heterogeneity (HKY-Γ) provided the best fit and was subsequently used. The statistical robustness of the phylogenetic trees was tested by bootstrapping with 1,000 replicates (bootstrap values of 70% were used as a cutoff point for cluster analysis; only branching with a bootstrap value of ≥70% was defined as robust clustering). For each HCV cluster, we estimated the year of origin of the most recent common ancestor (TMRCA) using a molecular clock approach, which places the phylogenetic history of HCV onto a timescale of months and years. On the basis of previous estimates, the inferred rate of nucleotide evolution of our NS5B sequences was 5.0 × 10−4 (18). The year of origin for each cluster was subsequently calculated by using an HKY model with a variable rate for each codon position, as implemented in the Tipdate program (21).
Statistical analysis.
Differences in epidemiological characteristics between HCV-4-positive patients allocated to phylogenetically distinct HCV-4 clusters were tested by the χ2 test in the case of sex, country of birth, most plausible route of HCV transmission, and HIV infection status and by analysis of variance in the case of age. The STATA statistical software package (StataCorp, College Station, TX) was used.
Nucleotide accession numbers and reference sequences.
The sequences reported in this study have been submitted to the GenBank database and can be retrieved under accession numbers FJ807047 through FJ807168.
The GenBank accession numbers of the full-genome reference sequences used to determine the HCV genotype were as follows: Y11604 (subtype 4a), FJ462435 (subtype 4b), FJ462436 (subtype 4c), DQ516083 (subtype 4d), EU392175 (subtype 4f), FJ462432 (subtype 4g), EU392171 (subtype 4k), FJ839870 (subtype 4l), FJ462433 (subtype 4m), FJ462441 (subtype 4n), FJ462440 (subtype 4o), FJ462431 (subtype 4p), FJ462434 (subtype 4q), FJ462439 (subtype 4r), and FJ839869 (subtype 4t). The GenBank accession numbers of the reference sequences of the HCV subtypes omitted from the list above were available only for NS5B fragment 1 and are as follows: L29590 (subtype 4e), L29611 (subtype 4h), L36437 (subtype 4i), and L36438 (subtype 4j).
RESULTS
Study population.
A combined search of the AMC and AHS databases identified 135 patients infected with HCV-4: 65 who had attended AMC, 48 who had attended AHS, and 22 who had attended both institutions. Two patients who had attended AMC were excluded when amplification and sequencing of the NS5B region revealed that they were infected with subtypes 1b and 1g. As shown in Table 1, the remaining 133 HCV-4-infected patients had a median age of 43 years (interquartile range [IQR], 36 to 48 years), 85% were male, 50% were born in Europe, and 28% were coinfected with HIV. The predominant underlying risk factors for infection were birth in a country where HCV is endemic (mostly Egypt and countries in Africa) (37%), injecting drug use (IDU; 32%), and high-risk sexual behavior (21%).
TABLE 1.
Epidemiological characteristics of study populationa
| Characteristic | No. of patients | % of patients |
|---|---|---|
| Sex | ||
| Female | 20 | 15.0 |
| Male | 113 | 85.0 |
| Ethnic origin | ||
| Dutch | 52 | 39.1 |
| European | 14 | 10.5 |
| Egyptian | 39 | 29.3 |
| African | 10 | 7.5 |
| Other | 12 | 9.0 |
| Unknown | 6 | 4.5 |
| Risk factors | ||
| IDU | 43 | 32.3 |
| High-risk sexual behavior | 27 | 20.3 |
| Transfusion | 4 | 3.0 |
| Birth in a country where HCV is endemic | 49 | 36.8 |
| Unknown | 10 | 7.5 |
| HIV coinfection | ||
| Positive | 37 | 27.8 |
| Negative | 75 | 56.4 |
| Not tested | 21 | 15.8 |
| HCV-4 subtype | ||
| 4a | 46 | 34.6 |
| 4d | 71 | 53.4 |
| 4h | 1 | 0.8 |
| 4k | 2 | 1.5 |
| 4n | 1 | 0.8 |
| 4o | 1 | 0.8 |
| 4r | 3 | 2.3 |
| 4 (unrecognized subtype) | 1 | 0.8 |
| No PCR fragment | 7 | 5.3 |
| Total | 133 | 100.0 |
The median age of the subjects was 43 years (IQR, 36 to 48 years).
Reverse transcription-PCR, sequencing, and genotyping.
Sera were available from 123/133 (93%) patients. Amplification and sequencing of NS5B fragment 1 succeeded in 119/123 (94%); the remaining 4 patients had HCV loads below 1,000 IU/ml. NS5B fragment 2 was obtained from 120/123 (98%) patients. Failure in one sample was due to a low viral load (<1,000 IU/ml), and in two samples, failure was most likely due to subtype-specific (HCV-4o and an unrecognized HCV-4 subtype) mutations at the primer locus. For four patients for whom serum samples were lacking, HCV NS5B fragment 2 sequencing data were available from an earlier study (31).
In total, the combined HCV NS5B fragment was obtained from 117/133 patients. Moreover, sequence data for at least one NS5B fragment were available for 126/133 patients. HCV genotyping of these 126 partial HCV NS5B sequences revealed eight distinct HCV-4 subtypes. The vast majority of patients were infected with HCV subtypes 4d (n = 71; 56%) and 4a (n = 46; 37%). The isolates from the remaining nine patients (6%) belonged to subtypes 4 h (n = 1), 4k (n = 2), 4n (n = 1), 4o (n = 1), 4r (n = 3), and a subtype not previously recognized (n = 1).
Phylogenetic analysis.
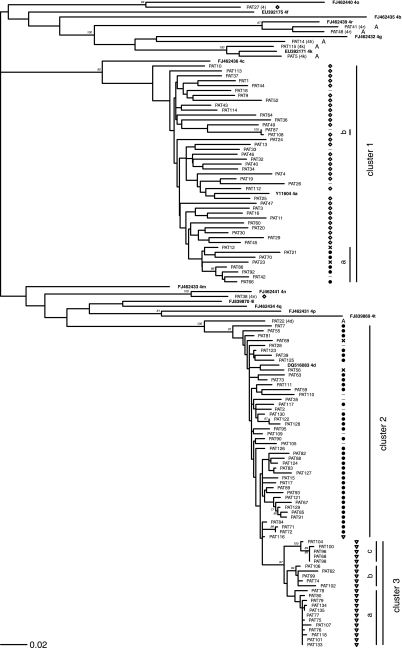
Phylogenetic trees were constructed for the HCV NS5B fragments combined and separately for fragment 1 and fragment 2. Figure 1 shows the HCV-4 phylogeny of the NS5B fragments combined. It represents the results for 117 HCV-4-infected patients and their assumed mode of HCV transmission, plus the results for 15 available HCV reference sequences. Phylogenetic analysis revealed three monophyletic clusters (bootstrap value, >70) containing 116/126 (92%) HCV-4-infected patients for whom a partial NS5B sequence was available. Table 2 summarizes the epidemiological characteristics of these patients according to the phylogenetic cluster to which they were allocated. The clusters were significantly different by sex, country of birth, HIV infection status, and plausible route of transmission (P < 0.001) but not by age (Table 2). Clearly, each cluster was linked to a specific epidemiological profile: immigrants from Egypt (cluster 1 [C1]), individuals reporting IDU (C2), and HIV-positive men who have sex with men (MSM) (C3).
FIG. 1.
HCV phylogenetic tree (combined NS5B fragments) and clustering of patients diagnosed with HCV-4 infection in the Amsterdam region. The risk factors allocated for each patient are listed opposite each sequence: born in Egypt (⋄), born in other areas where HCV is endemic area (A), IDU (•), high-risk sexual behavior of MSM (▿), blood transfusion in Europe before 1991 (×), and other or unknown (−).
TABLE 2.
Comparison of epidemiological characteristics of the patients in the distinct HCV-4 clusters in The Netherlandsa
| Characteristic | No. (%) of patients |
P value | ||
|---|---|---|---|---|
| C1 | C2 | C3 | ||
| Sex | <0.001 | |||
| Female | 2 (4) | 12 (27) | 0 | |
| Male | 44 (96) | 32 (73) | 26 (100) | |
| Country of birth | <0.001 | |||
| The Netherlands | 6 (13) | 25 (57) | 21 (81) | |
| Europe (excluding The Netherlands) | 2 (4) | 9 (20) | 3 (12) | |
| Egypt | 32 (70) | 0 (-) | 0 | |
| Other | 4 (9) | 7 (16) | 2 (8) | |
| Unknown | 2 (4) | 3 (7) | 0 | |
| Transmission route | <0.001 | |||
| IDU | 6 (13) | 35 (80) | 1 (4) | |
| Transfusion | 2 (4) | 2 (5) | 0 | |
| Sexual transmission, MSM | 0 | 1 (2) | 25 (96) | |
| Sexual transmission, FSWb | 1 (2) | 0 | 0 | |
| Exposure in a country where HCV is endemic | 34 (74) | 0 | 0 | |
| Unknown | 3 (7) | 6 (14) | 0 | |
| HIV coinfection | <0.001 | |||
| Positive | 4 (9) | 8 (18) | 24 (92) | |
| Negative | 32 (70) | 32 (73) | 2 (8) | |
| Not tested | 10 (22) | 4 (9) | 0 | |
| Total | 46 | 44 | 26 | |
The patients in C1 were infected with HCV-4 subtype 4a, and those in C2 and C3 were infected with HCV-4 subtype 4d. The median ages of the patients in C1, C2, and C3 were 43 years (IQR, 38 to 48 years), 39 years (IQR, 34 to 45 years), and 41 years (IQR, 36 to 48 years), respectively (P = 0.12).
FSW, female sex worker.
The largest cluster, C1, contained 46 sequences of HCV subtype 4a. The vast majority of the patients in this cluster were male (96%), of Egyptian descent (70%), and presumed to be HIV negative (91%). Within this so-called Egyptian cluster, phylogenetic analysis revealed one subcluster (C1a) of isolates from nine patients of non-Egyptian descent: six patients reporting IDU (four of them Dutch), two Dutch hemophiliacs, and one patient from Pakistan for whom information on the mode of transmission was missing. In addition, we identified a pair (C1b) of highly similar HCV-4a strains. These two strains were obtained from a female sex worker born in Suriname and a heterosexual HIV-positive Egyptian male who reported commercial sex contacts in The Netherlands.
Both C2 and C3 contained sequences of HCV subtype 4d. C2 comprised isolates from 44 patients; the majority were male (73%), reported IDU as the primary risk factor for HCV transmission (80%), and were of European descent (77%). In C2, 18% of the patients were coinfected with HIV. C3, which was actually a robust subcluster of C2, consisted of 26 sequences obtained from MSM who were diagnosed with acute HCV infection in The Netherlands after the year 2000. HIV-HCV coinfection was present in 24/26 (92%) of the MSM in C3; 1 MSM without HIV infection reported needle sharing during ketamine use and was therefore allocated to the IDU risk category.
In addition to these three clusters, phylogenetic analysis identified 10 unique unrelated isolates, all of which were from immigrants from areas in Africa or the Middle East where HCV is highly endemic. The median age of these 10 patients was 48 years (IQR, 38 to 60 years); 7 of them were male and 1 was coinfected with HIV. The isolates included three strains of HCV-4r (Somalia, Ethiopia); two strains of HCV-4k (Kenya, Rwanda); and one strain each of HCV-4d (Eritrea), HCV-4h (Democratic Republic of Congo), HCV-4n (Egypt), HCV-4o (Egypt), and a subtype not previously recognized (Egypt).
To gain additional insight into the spread of HCV-4 over time, we used a molecular clock approach to estimate the year of origin of TMRCA for each of the HCV-4 clusters identified (Table 3). For C1, TMRCA was traced back to 1881 (95% confidence interval [CI], 1833 to 1912), with 42/43 (98%) strains sharing one ancestor after 1909. Viral spread was reflected by the temporal distribution of lineage splits in each cluster. Within C1, 28/42 (67%) lineage splits occurred over the period from 1920 to 1955. Virtually no spread occurred after 1980, suggesting that cluster 1 reflects a random sample of HCV-4a strains imported by immigrants who acquired their infections in Egypt.
TABLE 3.
Analysis of most recent common ancestor in each cluster
| Cluster | Genotype | No. of sequences | Yr of origin (95% CI) | No. (%)b of lineage splits since: |
||
|---|---|---|---|---|---|---|
| 1980 | 1990 | 2000 | ||||
| C1a | 4a | 43 | 1881 (1833-1912) | 3 (7) | 1 (2) | 1 (2) |
| C2 | 4d | 44 | 1954 (1936-1965) | 12 (28) | 4 (9) | 1 (2) |
| C2, excluding patients 7, 28, 55, 69, and 81 | 4d | 39 | 1966 (1952-1974) | 12 (32) | 4 (11) | 1 (3) |
| C3a | 4d | 22 | 1985 (1977-1989) | 21 (100) | 19 (90) | 12 (57) |
| C3a | 4d | 12 | 1992 (1988-1995) | 11 (100) | 11 (100) | 9 (82) |
| C3b | 4d | 5 | 1994 (1989-1997) | 4 (100) | 4 (100) | 0 (0) |
| C3c | 4d | 5 | 1997 (1994-1999) | 4 (100) | 4 (100) | 3 (75) |
Calculations were based on data for patients for whom both PCR fragments were available. For C1, three patients lacked NS5B fragment 1; for C3, four patients lacked NS5B fragment 1.
The number of possible lineage splits per cluster (100%) is equivalent to the number of sequences in that cluster minus 1.
In contrast, the majority of patients infected with HCV-4d (C2 and C3) were of Dutch origin. Evolutionary analysis suggests the introduction of HCV-4d in Europe in 1954 (95% CI, 1936 to 1965). However, the exclusion of patients 7, 28, 55, 69, and 81, all of whom were born and/or infected in Italy or Spain and who represent old single branches within C2, suggests that HCV-4d was introduced in southern Europe before it reached The Netherlands somewhat later. On the basis of the temporal distribution of the lineage splits, 70% of the spread of HCV-4d strains among Dutch individuals reporting IDU occurred before 1980, and 90% occurred before 1990. The two percentages indicate a withdrawing epidemic, for which rapid transmission occurred in the past but for which the current incidence is low. MSM C3 originated from the same HCV-4d strain circulating among individuals reporting IDU, probably in about 1985 (95% CI, 1977 to 1989). However, C3 could be further subdivided into three distinct subclusters (C3a, C3b, C3c) that varied in origin from 1992 to 1997. The temporal distribution of the lineage splits of C3 suggests the rapid and ongoing transmission of HCV-4d among MSM, as 90% of the lineage splits occurred after 1990 and even 57% occurred after 2000.
DISCUSSION
The distribution of HCV-4 in the Amsterdam area showed a large phylogenetic diversity, with a total of eight subtypes (subtypes 4a, 4d, 4h, 4k, 4n, 4o, and 4r and an unrecognized subtype) being identified by phylogenetic analysis of the NS5B region. However, two subtypes, HCV-4a and HCV-4d, accounted for 93% of the HCV-4 infections in our population. Phylogenetic analysis revealed three distinct HCV-4 clusters, one of subtype 4a and two of subtype 4d, each of which had a specific epidemiological profile. We consider strains of HCV-4a to be imported, and they were found mainly among Egyptian immigrants who were infected in their home country. On the other hand, HCV-4d has become endemic in The Netherlands and circulates among individuals reporting IDU and HIV-positive MSM. Infections with all other HCV-4 subtypes were linked to immigrants from Africa and the Middle East.
The first epidemiological profile consists of immigrants from the Middle East and Africa, mainly Egyptian immigrants infected with HCV-4a in their home country. In the Amsterdam region, HCV-4a accounts for at least 35% of all HCV-4 infections. Egypt has the highest prevalence of HCV infections in the world due to the use of unsterile equipment during mass treatment of the population with parenteral antischistosomal therapy from the 1920s to the 1980s (7). Approximately 90% of Egyptian isolates belong to HCV-4a (22). Earlier analyses of the NS5B region of Egyptian HCV-4a isolates suggest that TMRCA existed 115 years ago (22), and due to parenteral antischistosomal therapy, these preexisting HCV strains underwent rapid exponential growth between 1924 and 1966 (20). The HCV-4a infections in The Netherlands most likely represent a random sample of HCV-4a strains imported from Egypt, given that evolutionary analysis of the HCV-4a strains in The Netherlands showed a TMRCA of 1881. Moreover, the vast majority of lineage splits occurred between 1924 and 1966, and 70% of patients were of Egyptian descent. In contrast, 94% of HCV-4a cases in southwestern France are of French origin, with IDU most often being reported as the probable mode of transmission (14). The evolutionary distances among French HCV-4a isolates are comparable to those observed among French HCV-4d isolates, suggesting the simultaneous introduction and endemic spread of both subtypes in the French IDU population. In Amsterdam, we did observe a small subcluster of nine highly related HCV-4a strains, mainly among Dutch individuals reporting IDU. Hence, in Amsterdam, as in France, some spillover of HCV-4a to the IDU scene has occurred, either directly through Egyptian immigration or indirectly through the migration of HCV-4a-infected individuals reporting IDU from regions such as those reported in France (14). Interestingly, a similar distinction between highly diverse HCV-4a strains from Egyptian immigrants and a cluster of highly related strains among individuals reporting IDU was observed in an urban area near Paris, France (12).
The second risk profile is linked to IDU, which accounts for an estimated 70% of newly acquired HCV infections in developed countries (1). In Europe, the prevalence of HCV-4d among HIV-HCV-coinfected individuals reporting IDU is relatively high, ranging from 7% in northern Europe to 24% in southern Europe (30). During the period from 1985 to 2005, approximately 10% of newly acquired HCV infections among individuals reporting IDU in Amsterdam were HCV-4, and virtually all were HCV-4d (34). The extent of homogeneity between HCV-4d strains across Europe suggests that genotype 4d entered the southern European IDU population relatively recently through just one or a few introductions (30). Liberal drug use legislation makes The Netherlands attractive to foreign individuals reporting IDU, whose movement within Europe could have brought HCV-4d to the Amsterdam region. Our evolutionary analysis of HCV-4d infections in Amsterdam indicates that HCV-4d entered the European IDU population as early as 1954, effectively spreading in the Amsterdam area since the 1960s. It followed the introduction and exponential growth of HCV-1a and HCV-3a, traditionally associated with IDU, which entered the European IDU population in the first half of the 20th century (19). Over the last two decades, the incidence of HCV infection among Dutch individuals reporting IDU has declined drastically, from 27.5/100 person years in the late 1980s to approximately 2/100 person years after 2000 (36), mainly due to decreased IDU combined with an effective harm reduction strategy in The Netherlands (35). This major decline is confirmed by the temporal distribution of the HCV-4d lineage splits that we found among individuals in Amsterdam reporting IDU: only 3/42 splits occurred after 1980. Nevertheless, the presence of chronic HCV carriers in The Netherlands and the less profound declines in the rates of HCV transmission among individuals reporting IDU in other European countries keep the prevalence of HCV infection high among individuals reporting IDU, varying from 50% in The Netherlands to 90% elsewhere in Europe (23, 36).
The third risk profile, also caused by HCV-4d, consists of MSM, nearly all of whom are coinfected with HIV. HCV is emerging as a sexually transmitted infection (STI) and is rapidly affecting increasing proportions of HIV-positive MSM in Europe (4, 8, 26, 29, 31). According to anonymous surveys conducted in 2007 and 2008, 15 to 21% of HIV-positive MSM attending the STI clinic in Amsterdam were coinfected with HCV, whereas the estimate was 1 to 4% before the year 2000 (29). Most of these HCV infections relate to permucosal risk factors in the context of (traumatic) sexual practices (4, 29). Rough sexual techniques and ulcerative STIs might favor the sexual transmission of HCV through mucosal lesions. The use of recreational drugs (even without injection) to enhance sexual pleasure and to remove inhibition during anal intercourse also enhances the likelihood of mucosal trauma or bleeding, especially when substances are applied anally (4, 26, 29, 31). In Amsterdam, more than 30% of HIV-HCV-coinfected MSM harbor a variant of the same HCV-4d strain (31). Since 0 to 18% of coinfected MSM in Europe report IDU (29, 31) and phylogenetic analysis confirms that the HCV-4d strain circulating among MSM originates from the one circulating among individuals reporting IDU, it is plausible that HCV entered the MSM population through its overlap with the IDU scene (29). The timescale of the HCV epidemic among HIV-infected MSM coincides with the introduction of highly active antiretroviral therapy (HAART) and the subsequent rise in the practice of high-risk sexual behavior and the incidence of STIs among MSM (4). Using evolutionary analysis, we calculated that 15/21 (76%) of the lineage splits in the MSM clade occurred after the introduction of HAART in 1996. However, two of three subclusters of HCV isolates among MSM arose before 1996, suggesting that the transfer of HCV from individuals reporting IDU to MSM preceded the introduction of HAART.
In conclusion, we observed three distinct monophyletic clusters of HCV-4 in Amsterdam that correspond to three distinct epidemiological profiles: import of HCV-4a mainly by immigrants from areas in Africa and the Middle East where HCV is endemic, the local spread of HCV-4d in individuals reporting IDU, and its spillover into HIV-positive MSM. Since infections with HCV-4 are difficult to treat and cause an estimated 10% of chronic HCV infections in The Netherlands (5, 32), effective therapy against HCV-4 will increasingly be needed. The need is heightened as HCV-4d rapidly spreads among HIV-positive MSM and becomes more prevalent in European individuals reporting IDU with and without HIV coinfection. Nevertheless, most clinical trials are focused on patients infected with HCV-1, despite the high worldwide prevalence of HCV-4 infections. In our opinion, clinical research and drug development programs must be stimulated to further deter the spread of HCV-4 infection.
Acknowledgments
We thank all participating physicians, nurses, and laboratory staff of the AHS and AMC, in particular, C. J. Weegink, S. Menting, and S. P. H. Rebers (AMC), as well as the head of the molecular biology department of the Laboratory of Public Health, S. M. Bruisten (AHS). We also thank M. Schim van der Loeff of the AHS for his statistical advice. Finally, the study could not have been performed without the cooperation of the study participants.
Footnotes
Published ahead of print on 30 September 2009.
REFERENCES
- 1.Alter, M. J. 2002. Prevention of spread of hepatitis C. Hepatology 36:S93-S98. [DOI] [PubMed] [Google Scholar]
- 2.Ansaldi, F., B. Bruzzone, S. Salmaso, M. C. Rota, P. Durando, R. Gasparini, and G. Icardi. 2005. Different seroprevalence and molecular epidemiology patterns of hepatitis C virus infection in Italy. J. Med. Virol. 76:327-332. [DOI] [PubMed] [Google Scholar]
- 3.Beld, M., R. Minnaar, J. Weel, C. Sol, M. Damen, H. van der Avoort, P. Wertheim-van Dillen, A. van Breda, and R. Boom. 2004. Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription-PCR with an armored RNA internal control. J. Clin. Microbiol. 42:3059-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danta, M., D. Brown, S. Bhagani, O. G. Pybus, C. A. Sabin, M. Nelson, M. Fisher, A. M. Johnson, and G. M. Dusheiko. 2007. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS 21:983-991. [DOI] [PubMed] [Google Scholar]
- 5.Delwaide, J., C. Reenaers, C. Gerard, D. Vaira, B. Bastens, B. Servais, A. Bekhti, C. Bataille, E. Wain, P. De Leeuw, et al. 2006. HCV genotype 4 in Belgium: three distinct patterns among patients from European and African origin. Eur. J. Gastroenterol. Hepatol. 18:707-712. [DOI] [PubMed] [Google Scholar]
- 6.de Vries, M. J., B. te Rijdt, and C. M. van Nieuwkerk. 2006. Genotype distribution amongst hepatitis C patients in The Netherlands. Neth. J. Med. 64:109-113. [PubMed] [Google Scholar]
- 7.Frank, C., M. K. Mohamed, G. T. Strickland, D. Lavanchy, R. R. Arthur, L. S. Magder, T. El Khoby, Y. Abdel-Wahab, E. S. Aly Ohn, W. Anwar, and I. Sallam. 2000. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 355:887-891. [DOI] [PubMed] [Google Scholar]
- 8.Giraudon, I., M. Ruf, H. Maguire, A. Charlett, F. Ncube, J. Turner, R. Gilson, M. Fisher, S. Bhagani, M. Johnson, and S. Barton. 2008. Increase in diagnosed newly acquired hepatitis C in HIV-positive men who have sex with men across London and Brighton, 2002-2006: is this an outbreak? Sex Transm. Infect. 84:111-115. [DOI] [PubMed] [Google Scholar]
- 9.Guindon, S., and O. Gascuel. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696-704. [DOI] [PubMed] [Google Scholar]
- 10.Kamal, S. M., and I. A. Nasser. 2008. Hepatitis C genotype 4: what we know and what we don't yet know. Hepatology 47:1371-1383. [DOI] [PubMed] [Google Scholar]
- 11.Lauer, G. M., and B. D. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41-52. [DOI] [PubMed] [Google Scholar]
- 12.Morice, Y., D. Roulot, V. Grando, J. Stirnemann, E. Gault, V. Jeantils, M. Bentata, B. Jarrousse, O. Lortholary, C. Pallier, and P. Deny. 2001. Phylogenetic analyses confirm the high prevalence of hepatitis C virus (HCV) type 4 in the Seine-Saint-Denis District (France) and indicate seven different HCV-4 subtypes linked to two different epidemiological patterns. J. Gen. Virol. 82:1001-1012. [DOI] [PubMed] [Google Scholar]
- 13.Murphy, D. G., B. Willems, M. Deschenes, N. Hilzenrat, R. Mousseau, and S. Sabbah. 2007. Use of sequence analysis of the NS5B region for routine genotyping of hepatitis C virus with reference to C/E1 and 5′ untranslated region sequences. J. Clin. Microbiol. 45:1102-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicot, F., F. Legrand-Abravanel, K. Sandres-Saune, A. Boulestin, M. Dubois, L. Alric, J. P. Vinel, C. Pasquier, and J. Izopet. 2005. Heterogeneity of hepatitis C virus genotype 4 strains circulating in south-western France. J. Gen. Virol. 86:107-114. [DOI] [PubMed] [Google Scholar]
- 15.Nylander, J. A., F. Ronquist, J. P. Huelsenbeck, and J. L. Nieves-Aldrey. 2004. Bayesian phylogenetic analysis of combined data. Syst. Biol. 53:47-67. [DOI] [PubMed] [Google Scholar]
- 16.Payan, C., F. Roudot-Thoraval, P. Marcellin, N. Bled, G. Duverlie, I. Fouchard-Hubert, P. Trimoulet, P. Couzigou, D. Cointe, C. Chaput, C. Henquell, et al. 2005. Changing of hepatitis C virus genotype patterns in France at the beginning of the third millennium. J. Viral Hepat. 12:405-413. [DOI] [PubMed] [Google Scholar]
- 17.Poynard, T., M. F. Yuen, V. Ratziu, and C. L. Lai. 2003. Viral hepatitis C. Lancet 362:2095-2100. [DOI] [PubMed] [Google Scholar]
- 18.Pybus, O. G., M. A. Charleston, S. Gupta, A. Rambaut, E. C. Holmes, and P. H. Harvey. 2001. The epidemic behavior of the hepatitis C virus. Science 292:2323-2325. [DOI] [PubMed] [Google Scholar]
- 19.Pybus, O. G., A. Cochrane, E. C. Holmes, and P. Simmonds. 2005. The hepatitis C virus epidemic among injecting drug users. Infect. Genet. Evol. 5:131-139. [DOI] [PubMed] [Google Scholar]
- 20.Pybus, O. G., A. J. Drummond, T. Nakano, B. H. Robertson, and A. Rambaut. 2003. The epidemiology and iatrogenic transmission of hepatitis C virus in Egypt: a Bayesian coalescent approach. Mol. Biol. Evol. 20:381-387. [DOI] [PubMed] [Google Scholar]
- 21.Rambaut, A. 2000. Estimating the rate of molecular evolution: incorporating non-contemporaneous sequences into maximum likelihood phylogenies. Bioinformatics 16:395-399. [DOI] [PubMed] [Google Scholar]
- 22.Ray, S. C., R. R. Arthur, A. Carella, J. Bukh, and D. L. Thomas. 2000. Genetic epidemiology of hepatitis C virus throughout Egypt. J. Infect. Dis. 182:698-707. [DOI] [PubMed] [Google Scholar]
- 23.Roy, K., G. Hay, R. Andragetti, A. Taylor, D. Goldberg, and L. Wiessing. 2002. Monitoring hepatitis C virus infection among injecting drug users in the European Union: a review of the literature. Epidemiol. Infect. 129:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Quijano, A., M. A. Abad, R. Torronteras, C. Rey, J. A. Pineda, M. Leal, J. Macias, and E. Lissen. 1997. Unexpected high prevalence of hepatitis C virus genotype 4 in southern Spain. J. Hepatol. 27:25-29. [DOI] [PubMed] [Google Scholar]
- 25.Schroter, M., B. Zollner, P. Schafer, A. Reimer, M. Muller, R. Laufs, and H. H. Feucht. 2002. Epidemiological dynamics of hepatitis C virus among 747 German individuals: new subtypes on the advance. J. Clin. Microbiol. 40:1866-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serpaggi, J., M. L. Chaix, D. Batisse, C. Dupont, A. Vallet-Pichard, H. Fontaine, J. P. Viard, C. Piketty, E. Rouveix, C. Rouzioux, L. Weiss, and S. Pol. 2006. Sexually transmitted acute infection with a clustered genotype 4 hepatitis C virus in HIV-1-infected men and inefficacy of early antiviral therapy. AIDS 20:233-240. [DOI] [PubMed] [Google Scholar]
- 27.Simmonds, P., J. Bukh, C. Combet, G. Deleage, N. Enomoto, S. Feinstone, P. Halfon, G. Inchauspe, C. Kuiken, G. Maertens, et al. 2005. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 42:962-973. [DOI] [PubMed] [Google Scholar]
- 28.Smith, D. B., S. Pathirana, F. Davidson, E. Lawlor, J. Power, P. L. Yap, and P. Simmonds. 1997. The origin of hepatitis C virus genotypes. J. Gen. Virol. 78(Pt 2):321-328. [DOI] [PubMed] [Google Scholar]
- 29.Urbanus, A. T., T. J. van de Laar, I. G. Stolte, J. Schinkel, T. Heijman, R. A. Coutinho, and M. Prins. 2009. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS 23:F1-F7. [DOI] [PubMed] [Google Scholar]
- 30.van Asten, L., I. Verhaest, S. Lamzira, I. Hernandez-Aguado, R. Zangerle, F. Boufassa, G. Rezza, B. Broers, J. R. Robertson, R. P. Brettle, et al. 2004. Spread of hepatitis C virus among European injection drug users infected with HIV: a phylogenetic analysis. J. Infect. Dis. 189:292-302. [DOI] [PubMed] [Google Scholar]
- 31.van de Laar, T., O. Pybus, S. Bruisten, D. Brown, M. Nelson, S. Bhagani, M. Vogel, A. Baumgarten, M. L. Chaix, M. Fisher, et al. 2009. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 136:1609-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Laar, T. J., M. H. Koppelman, A. K. van der Bij, H. L. Zaaijer, H. T. Cuijpers, C. L. van der Poel, R. A. Coutinho, and S. M. Bruisten. 2006. Diversity and origin of hepatitis C virus infection among unpaid blood donors in The Netherlands. Transfusion 46:1719-1728. [DOI] [PubMed] [Google Scholar]
- 33.van de Laar, T. J., M. W. Langendam, S. M. Bruisten, E. A. Welp, I. Verhaest, E. J. van Ameijden, R. A. Coutinho, and M. Prins. 2005. Changes in risk behavior and dynamics of hepatitis C virus infections among young drug users in Amsterdam, The Netherlands. J. Med. Virol. 77:509-518. [DOI] [PubMed] [Google Scholar]
- 34.van de Laar, T. J., R. Molenkamp, C. van den Berg, J. Schinkel, M. G. Beld, M. Prins, R. A. Coutinho, and S. M. Bruisten. 2009. Frequent HCV reinfection and superinfection in a cohort of injecting drug users in Amsterdam. J. Hepatol. 51:667-674. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg, C., C. Smit, G. Van Brussel, R. Coutinho, and M. Prins. 2007. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction 102:1454-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Berg, C. H., C. Smit, M. Bakker, R. B. Geskus, B. Berkhout, S. Jurriaans, R. A. Coutinho, K. C. Wolthers, and M. Prins. 2007. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. Eur. J. Epidemiol. 22:183-193. [DOI] [PMC free article] [PubMed] [Google Scholar]