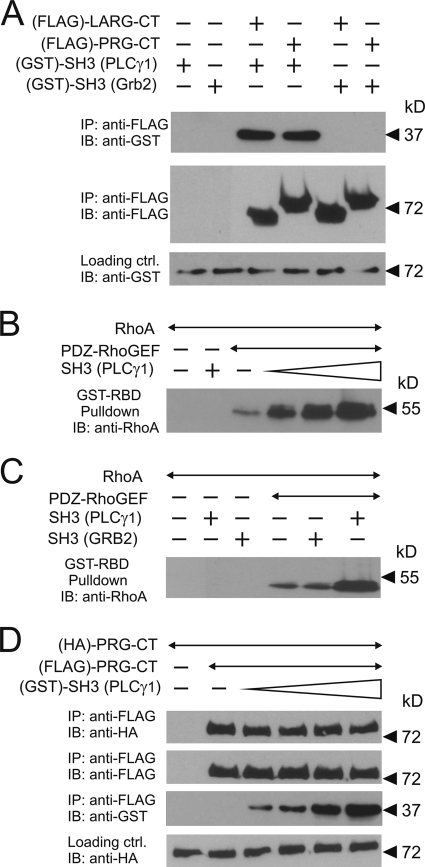
FIG. 7.
In vitro characterization of the role of the SH3 domain of PLCγ in PDZ-RhoGEF signaling. (A) Equal amounts of purified GST-tagged SH3 domains of PLCγ1 or Grb2 were incubated without (−) or with (+) purified FLAG-tagged C-terminal portions of LARG or PDZ-RhoGEF (LARG-CT and PRG-CT, respectively). The RhoGEF proteins were then immunoprecipitated (IP) using anti-FLAG antibody. Shown are immunoblots (IB) of the precipitates using the indicated antibodies. (B) One nanomole of FLAG-PDZ-RhoGEF was incubated without or with a 1-, 10-, or 100-fold molar excess of GST-SH3 of PLCγ1. The reaction was started by addition of 100 nanomole of GDP-loaded RhoA. After 10 min of incubation, activated RhoA was precipitated as described in Materials and Methods. Shown are immunoblots using the anti-RhoA antibody. (C) FLAG-PDZ-RhoGEF was incubated with the SH3 domains of PLCγ1 or Grb2. The experiment was performed as described in B. (D) Equal amounts of hemagglutinin (HA)- and FLAG-tagged C-terminal portions of PDZ-RhoGEF were incubated alone or together with increasing concentrations of the SH3 domain of PLCγ1. FLAG-tagged PDZ-RhoGEF was immunoprecipitated, and precipitates were analyzed by immunoblotting with the indicated antibodies.