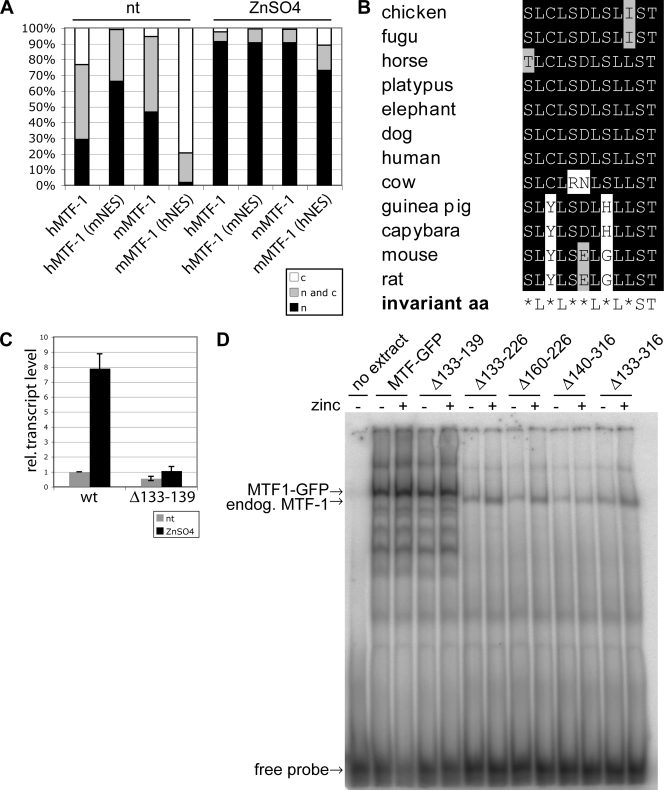
FIG. 4.
Mutations in MTF-1 suggest a link between export and metal inducibility. (A) To analyze the subcellular localization of the chimeric constructs, MTF-1 null cells (dko7) were transfected with wild-type or mutated MTF-1-VSV of mouse or human. Cells were stained using an anti-VSV antibody. Two hundred cells each were counted and classified into the indicated categories (c, cytoplasm; n, nucleus; n and c, equally distributed). The mutant with impaired metal induction showed increased nuclear localization, and the mutant with high metal inducibility showed increased cytoplasmic localization. mMTF-1, mouse MTF-1; nt, not treated. (B) Sequence comparison of the MTF-1 NESs of different species (corresponding to aa 335 to 346 of hMTF-1). The human sequence differs from the rodent sequences in 2 and 3 aa, respectively. The leucine residues, characteristic for NESs, are well conserved. (C) The transcriptional activity of MTF-1 with a mutation outside the NES that is impaired in nuclear export was analyzed by an S1 nuclease protection assay. This mutant is not metal inducible. Error bars indicate standard deviations of data from three independent experiments. wt, wild type. (D) Test for DNA binding by EMSA. HEK293 cells were transfected with MTF-1-GFP constructs as indicated and treated with 100 μM zinc sulfate or left untreated. MTF-1-GFP and MTF-1-GFP(Δ133-139) bind to the MRE-containing oligonucleotide, while the constructs with larger deletions did not. The band visible in the latter case is the band shift from endogenous (endog.) MTF-1 from HEK293 cells, which migrates faster because it lacks the GFP moiety.