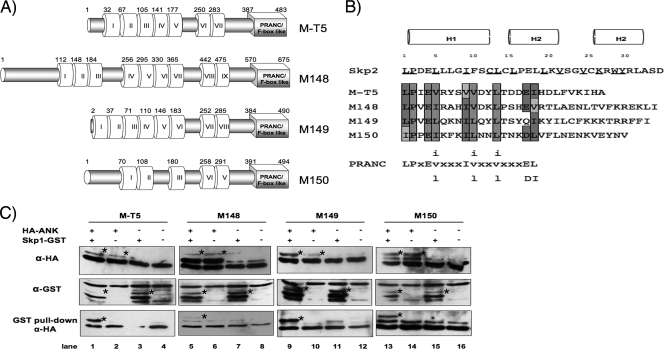
FIG. 1.
MYXV ANK repeat proteins include a functional C-terminal PRANC domain that binds Skp1. (A) Schematic representation of the four ANK repeat-containing proteins encoded by MYXV. Predicted ANK repeats and putative carboxyl-terminal PRANC/F-box-like domains are represented by white and gray boxes, respectively (24, 41). The first amino acid residue of each predicted ANK repeat and amino acid length of the corresponding proteins are indicated. (B) Alignment of the amino acid sequences of the carboxy-terminal region of each MYXV ANK repeat protein. A consensus PRANC/F-box-like sequence adapted from Mercer et al. (30) is shown with lowercase letters representing variant positions, and X indicates nonconserved positions. Dark-shaded letters represents residues identical to the poxviral consensus motif, while conserved substitutions are denoted with light shading. Nonconserved differences contain no shading. The F-box α-helical secondary structures are represented by H1, H2, and H3 (37, 63). Known residues of Skp2 that contact the linker protein Skp1 are underlined (40). (C) In vitro coupled transcription-translation was used to express the indicated (+) combination of plasmids, which include Skp1-GST and HA-tagged MYXV ANK repeat proteins M-T5, M148, M149, and M150. Samples were subjected to a GST pull-down assay. Precipitates and total lysates were resolved by SDS-PAGE and probed with anti-HA antibody to detect coprecipitated viral proteins (bottom row). Expression of MYXV ANK repeat proteins (top row) and Skp1 (middle row) was confirmed by immunoblotting with antibody against HA (anti-HA) and GST (anti-GST) epitopes, respectively. Bands of interest are indicated with stars. α, anti.