Abstract
The role of regulatory T cells (Tregs) in mediating immune suppression of anti-tumor immune responses is increasingly becoming appreciated in patients with malignancies - especially within the malignant glioma patient population. This review will discuss the role and prognostic significance of Tregs within glioma patients and will delineate potential approaches for their inhibition that can be used either alone or in combination with other immune therapeutics in clinical trials and in the clinical settings of recurrent and/or residual disease.
Keywords: regulatory T cells, glioblastoma multiforme, central nervous system, immunotherapy, vaccine, prognosis
Overview of immunosuppressive Tregs
The function of the immune system is to recognize foreign materials in the body and distinguish them from normal body tissues and cells. Immune responses consist of cell-mediated (T cells, natural killer cells, and phagocytes) and/or humoral (B cells, antibodies, and complement) responses modified and regulated by cytokines. Antigen-presenting cells (APCs) such as dendritic cells (DCs) and macrophages take up antigens, partially degrade them, and present them to T cells in the context of major histocompatibility complex (MHC) molecules. To fully activate the adaptive immune response, the T cells must receive two signals: one through the T cell receptor and the other through the co-stimulatory receptor CD28, which recognizes the co-stimulatory molecules CD80 and CD86 expressed on the surface of APCs. Failure to do so will result in T cell anergy [1]. T cells have a number of functions, including potentiating cytotoxic T cell responses (CD4+ helper T cells [Th1 cells]), assisting B cells in the production of antibodies (CD4+ helper T cells [Th2 cells]), recognizing and destroying virally infected or tumor cells (CD8+ effector T cells), and determining the level of reactivity in the immune system (CD4+/CD25+/[forkhead box P3+] Foxp3+ regulatory T cells [Tregs]).
Tregs are a physiologic subset of CD4+ T cells that curtail the function of T cells, B cells [2,3], DCs [4-6], monocytes and/or macrophages [6], natural killer T cells [7], and natural killer cells [8,9]. Tregs potently inhibit T cell cytokine secretion and proliferation by down regulating interleukin (IL)-2 and interferon (IFN)-γ production [10-14], increase Th2 cytokine skewing [15], directly curtail the generation and expansion of endogenous and/or induced immune responses by suppressing proinflammatory cytokine production [16-24], and apparently play a significant role in hindering immunity to tumor-associated antigens [25,26]. Furthermore, studies of murine models of immunogenic tumors have shown that adoptively transferred Tregs inhibit tumor-reactive effector T cells and that elimination of Tregs in vivo enhances antitumor immunity [15]. More specifically, in murine tumor challenge models of established gliomas, in vivo depletion of Tregs has resulted in enhanced tumor rejection and increased median survival durations [27-29].
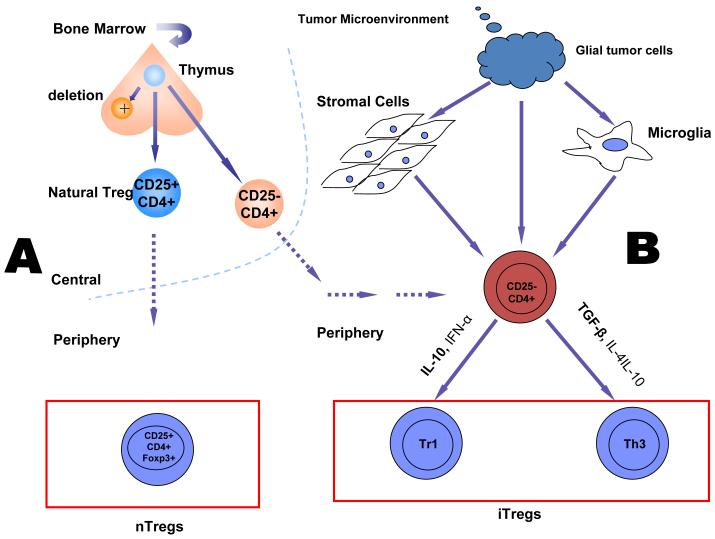
Since the discovery of Tregs [30], our understanding of the types and functions of these cells has increased greatly. Tregs are classified into three subtypes based on their induction site, cytokine profile, and respective cell surface markers: natural Tregs (nTregs), Th3 cells, and Tr1 cells (Fig. 1) [31-34]. nTregs (CD4+/CD25+/Foxp3+) are thymically derived, bind with intermediate affinity to the MHC/peptide complex, and are capable of recognizing both self-generated and foreign antigens. Upon their exportation to the periphery from the thymus, nTregs exert their effect on peripheral effector T cells primarily via cell-to-cell contact. Although the mechanism by which nTregs exert their effects on the effector T cells has yet to be fully elucidated [31-34], it likely results from downregulation of IL-2 cytokine production and may involve membrane-bound cytotoxic T lymphocyte antigen-4 (CTLA-4), a negative regulator of T cell activation, a member of the CD28/B7 immunoglobulin superfamily that is constitutively expressed on Tregs [35], and whose expression is up-regulated in activated T cells [36,37]. . The development of nTregs is regulated by the Foxp3 gene in CD4+/CD25+ T cells [38]. The primary role of nTregs is suspected to be maintenance of a constant homeostatic balance by curtailing the effects of autoreactive T cells in noninflammatory settings [32].
Fig. 1.
Schematic illustration of Treg development. (A) Illustration of the development of nTregs. nTregs are selected with an intermediate affinity for the MHC II/self-peptide complex in the thymus with an intermediate affinity for the MHC II/self-peptide complex. They then enter the peripheral circulation as CD4+/CD25+/Foxp3+ cells. The nTregs exert their effect on immune effector cells primarily via cell-to-cell contact both peripherally and within the tumor microenvironment. (B) Illustration of the development of iTregs. Tr1 and Th3 cells fall under the rubric of iTregs and are also derived from the thymus; however, they enter the peripheral circulation as naïve (CD4+/CD25-/Foxp3-) T cells. They are then induced in the periphery to differentiate into regulatory T cells via a myriad of redundant pathways emanating from the tumor microenvironment. Differentiation of naïve CD4+ cells into Tr1 and Th3 cells is cytokine-dependent, as is the mechanism by which the iTregs exert their effects on immune effector cells.
Tregs in circulating peripheral blood include not only nTregs differentiated in the thymus but also Foxp3+ Tregs generated extrathymically by the conversion of naïve T cells via chronic encounters with antigens present in suboptimal doses [39] or by the suppressive cytokine milieu secreted by the glioma. In vitro experiments have demonstrated that peripheral T cells retain the ability to induce Foxp3 expression upon T cell receptor cross-linking in the presence of TGF-β [40] or by the CD28/B7 interaction [41]. However, the overall contribution of the peripheral conversion of Tregs to immune suppression and its functional significance are not clear. Investigators have described two populations of peripherally induced CD4+ Tregs: Tr1 and Th3 cells. Tr1 cells require IL-10 for induction, predominantly secrete IL-10, and to a lesser degree TGF-β and INF-γ. Tr1 cells inhibit naïve, memory, and helper T cells and the ability of DCs to induce T cell proliferation [42]. In contrast, Th3 cells [43] are induced by IL-10 and TGF-β [44] and predominantly secrete TGF-β and IL-10 at levels lower than Tr1 cells do. It is suspected that these induced Tregs (iTregs) play a primary role in mitigating pathological immune responses such as those seen in cases of infection and autoimmune-mediated inflammation [32].
Biological role of Tregs in patients with glioma
Studies have implicated Tregs mediate immunosuppression in patients with a number of different malignancies, including ovarian, pancreatic, breast, colorectal, lung, and esophageal cancer [25,45,46]. For example, Curiel et al. [25] showed that the Treg fraction was higher in ascites of patients with ovarian cancer compared to that of patients with nonmalignant ascites. The authors also showed that Tregs preferentially migrated to the tumor microenvironment induced by CCL22 secreted by the tumor cells and macrophages. Furthermore, Tregs inhibited the function of tumor-infiltrating T cells by inhibiting production of IL-12 and INF-γ. These findings were instrumental in implicating a role for Tregs in tumor-mediated immunosuppression.
Patients with malignant gliomas have severe defects in host humoral and cellular immune responses [47]. These defects are characterized by dramatic reductions in both CD4+ T cell number [27] and function [48,49] and a disproportionate presence of immunosuppressive Tregs [27]. This increase in Treg fractions corresponds with a decrease in effector T cell functions. Furthermore, the removal of the Treg fraction from T cells obtained from patients with glioblastoma multiforme (GBM) restores T cell proliferation and cytokine responses to normal levels [27]. Moreover, in vitro depletion of Tregs from peripheral blood results in the successful reversal of effector T cell function, including increased T cell proliferation and a switch from Th2 to a Th1 (IL-2+/tumor necrosis factor-α (TNF-α)+/IFN-γ) cytokine profile. These findings demonstrate the important role of Tregs in glioma-mediated immunosuppression.
In the glioma microenvironment, the anti-tumor effector T cells can be critically suppressed and/or overwhelmed by Tregs. Researchers have obtained human glioma tissue during surgery, dissociated the tumors into single-cell suspensions, and stained them for the CD8+ and CD4+ subsets of T cells [50]. They found that tumor-infiltrating CD8+ T cells were phenotypically CD8+ and CD25-, indicating that these effector cells were not activated or proliferating. The CD4+ T cells were more numerous than CD8+ T cells within the gliomas, and the majority of CD4+ T cells were Tregs as evidenced by positive intracellular staining for Foxp3. In another study, the CD4+/CD25+/Foxp3+ T cells were found only in gliomas, whereas Tregs were absent from control brain tissue specimens [51]. The presence of Tregs within the glioma microenvironment is secondary to the elaboration of the chemokine CCL2 by gliomas which induce the migration of Tregs [52]. Finally, in murine models of syngeneic murine glioma, investigators have observed a time-dependent accumulation of Foxp3+ Tregs in brain tumors [53]. These data indicate that Tregs can not only inhibit the initial systemic anti-tumor immune activation but also prevent effector T cell responses in the tumor microenvironment and thus are a potential therapeutic target for inhibition.
Prognostic significance of Tregs in patients with glioma
T cells in the central nervous system (CNS) of healthy humans are a rare finding. However, during inflammatory responses, T cells are evident within the CNS. T cells require activation prior to entry into the CNS [54], but antigen specificity is not necessary for this entry. T cell infiltrates are commonly identified in human gliomas [55], and multiple studies have attempted to correlate the intensity of T cell infiltration with survival [55-57]; however, this prognostic significance has not been seen consistently [58]. Of note is that these types of immunohistochemical assays used in the aforementioned studies do not take into account the functional activity of these T cells or the influence of the immune inhibitory Tregs. Thus, although these T cells are activated in the systemic circulation, their functional activity likely becomes impaired upon entry into the glioma microenvironment [59]; thus, the fact that the T cells presence in a glioma is not a definitive prognostic marker is not surprising.
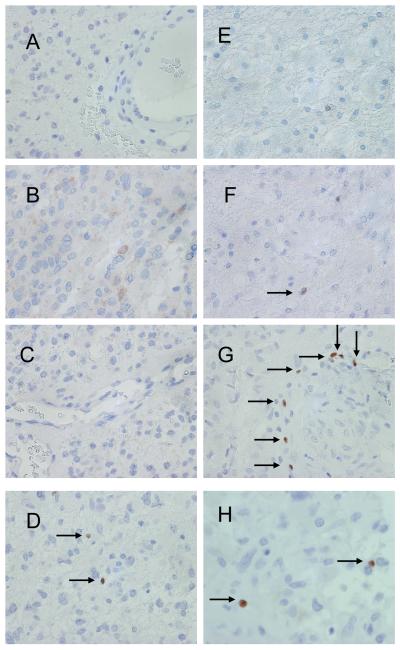
Researchers showed that CD8+ T cells were present in the majority of glioma specimens regardless of tumor grade; however, the number of patients with CD4+ T cell populations (including Tregs) increased as the tumor grade increased (39% for World Health Organization [WHO] grade II tumors to 73% for WHO grade III tumors to 98% for grade WHO grade IV tumors) [60]. Foxp3+ Tregs are not usually seen in normal brain tissue specimens and are very rare in patients with oligodendroglioma (WHO grade II), mixed oligoastrocytoma (WHO grade II), or anaplastic oligodendroglioma (WHO grade III) (Fig. 2). In contrast, 39% of the anaplastic mixed oligoastrocytoma specimens (WHO grade III), 53% of the anaplastic astrocytoma specimens (WHO grade III), 48% of the GBM specimens (WHO grade IV), and 83% of the gliosarcoma specimens (WHO grade IV) had Foxp3+ Tregs [60]. Thus, Tregs were more common in high-grade astrocytic gliomas than in low-grade oligodendroglioma-type tumors.
Fig. 2.
Immunohistochemical staining of human tissue sections for Foxp3 in (A) oligodendroglioma, (B) mixed oligoastrocytoma, (C) anaplastic oligodendroglioma, (D) anaplastic mixed oligodendroglioma, (E) low-grade astrocytoma, (F) anaplastic astrocytoma, (G) glioblastoma, and (H) gliosarcoma. Tissue sections for Foxp3 show faint staining with few nonclustering Tregs (B, D, F). The Treg number increased as the tumor grade increased (E-G), although they were nearly absent from tumors with oligo-based histologies (A and C). All of the images were taken at a magnification of 400x.
Because the presence of Foxp3+ Tregs correlates with the overall malignant behavior of astrocytic tumors, the expectation that the presence of Tregs in the tumor microenvironment will act as a negative prognostic indicator is reasonable. Univariate analysis demonstrated that, similar to other established parameters, such as the Karnofsky performance score, patient age, and tumor grade, the presence or absence of Foxp3+ Tregs and absolute number of Foxp3+ Tregs per tumor sample were prognostic factors. However, a multivariate analysis performed to account for confounding factors, such as patient age and Karnofsky performance score, found that the presence of Foxp3 Tregs did not have a prognostic impact [60]. Although some cancers may mediate immunosuppression predominantly via Tregs, high-grade gliomas have multiple mechanisms of mediating immunosuppression [47]. Thus, the lack of a prognostic impact of one mechanism in this setting, such as the presence or absence of Foxp3+ Tregs, is not entirely surprising and emphasizes the redundancy of immunosuppressive pathways. Furthermore, this type of study does not account for the prognostic influence of Tregs in the systemic, peripheral blood compartment.
Mechanisms of modulating Treg responses
It is widely recognized that tumors evade the host immune response through a number of mechanisms, including elaboration of immunosuppressive cytokines, alteration of signal transduction [61], and the induction of Tregs. Thus, at the core of the development of more effective immunotherapeutic strategies for brain tumors is simultaneous stimulation of more potent immune responses against these tumors while overcoming immunosuppressive mechanisms induced by the tumors themselves. Overcoming Treg-induced immunosuppression can be achieved using a variety of approaches, including administration of denileukin diftitox (Ontak; a recombinant protein of diphtheria toxin and IL-2) [62], cyclophosphamide (CTX) [63], an anti-CD25 antibody (targeting the IL-2 receptor) [29], CTLA-4 blockade (inhibits co-stimulation) [28], and signal transcription and activator of translation (STAT)-3--blocking agents that also block transcriptional activation of Foxp3 [64,65]; inhibition of intratumoral Treg trafficking (i.e., inhibition of CCL2) with temozolomide [66]; and, nonspecifically, lymphodepletion to augment immunological responses, which investigators have described in both murine models [63,67] and human patients with cancer [68,69]. Antitumor responses enhanced by lymphodepletion may be secondary to the removal of competition at the surface of APCs [70], enhanced availability of cytokines that augment T cell activity (such as IL-7 and IL-15) [71], and/or the depletion of immune inhibitory Tregs [58].
Developing an optimal approach to modulating or suppressing the Treg population for therapeutic purposes in cancer patients is an area of controversy. CTX, an alkylating agent with therapeutic effects against tumors at high doses, preferentially inhibits Tregs at lower doses. CTX can abolish the function of CD4+/CD25+/Foxp3+ T cells and enhance cytotoxic T cell responses [72]. Treatment with CTX prior to antitumor vaccination results in activation of tumor-specific CD8+ T cells [73]. When CTX is administered at subtumoricidal doses in combination with IL-12 in mice, it improves immune response and eradicates of large established sarcomas [74]. This combination enhances CD4+ T cells, CD8+ T cells, and macrophage infiltration in tumors and skews the immune responses to the Th1 phenotype [74]. Other investigators have reported similar findings of treatment of established melanomas with IL-2, IL-1, and CTX in mice [68]. In addition to its induction of immunity to new antigens, CTX can overcome immune tolerance. For example, administration of CTX in mice bearing established plasmacytomas resulted in a cure in 92% of the mice, and further studies demonstrated that the cured mice experienced rejection of a subsequent tumor challenge [75,76]. The mechanisms of efficacy appeared to include both generation of CD8+ cytotoxic T cells and up-regulation of expression of B7-1 (a co-stimulatory molecule).
Multiple clinical trials have demonstrated enhanced immune responses and improved clinical efficacy when CTX was administered prior to immunotherapy [77,78]. For example, when CTX was administered to augment an autologous melanoma vaccine in patients with metastatic melanoma, there were enhanced delayed-type hypersensitivity responses [79]. Researchers have seen similar potentiation of immune responses in metastatic breast cancer and renal cell carcinoma cases. CTX has also been used in a phase I clinical trial of glioma patients that demonstrated evidence of clinical efficacy [80]. In this trial, Plautz and colleagues gave a single dose of CTX to patients with newly diagnosed glioma prior to administration of adoptively transferred T cells harvested from the patients’ lymph nodes. They conducted this study without the benefit of our current understanding of the influence of CTX on Tregs; thus, the contribution of CTX to the clinical efficacy of this immune therapeutic strategy is unknown. Despite the use of CTX in clinical trials, the timing and dosing of CTX for optimal Treg inhibition [81,82] relative to each type of vaccination or immunotherapy strategy still needs further refinement.
Researchers have also studied temozolomide, which is capable of suppressing Tregs, in clinical trials of immunotherapy for GBM. Temozolomide is the standard of care for GBM and an alkylating agent that causes cell death by inducing cell-cycle arrest at G2/M phase and, likely, autophagy without apoptosis [83]. In addition to inhibiting the proliferation of lymphocytes, temozolomide can deplete Tregs [66,84] and inhibit trafficking of Tregs into the glioma microenvironment [52]. The use of temozolomide has proven to be beneficial in combination with a peptide vaccine targeting epidermal growth factor receptor variant III in a phase II clinical trial in patients with newly diagnosed GBM [85]. Another clinical trial, sponsored by Northwest Biotherapeutics (Bethesda, MD), is using GBM patient autologous DCs for an immunotherapy in combination with temozolomide in a similar manner. The use of temozolomide as a possible Treg modulator has particular appeal in the glioma patient population since it is the current standard of care.
Inactivation of Tregs by treatment with an anti-CD25 antibody [29,53,86] or CTLA-4 blockade [46,53] have demonstrated efficacy in murine glioma models. Various studies support the notion that CTLA-4 blockade can enhance antitumor immune responses by limiting suppression of effector T cell responses [87] either by directly activating effector T cells or indirectly inactivating Tregs. Although some studies have shown that CTLA-4 blockade fails to suppress Tregs [87], others have indicated that Tregs and CTLA-4 blockade act independently and that the effects of CTLA-4 blockade are not focused on Tregs [35,88,89], such that CTLA-4 blockade may be synergistic with strategies that inhibit Tregs [89]. In one study of systemic delivery of CTLA-4 blocking monoclonal antibodies in mice with well-established malignant astrocytomas recapitulating the biology of human gliomas [90], the treatment produced a long-term survival rate of 80% without induction of autoimmune encephalomyelitis [28]. CTLA-4 blockade also re-established normal CD4+ T cell counts and abrogated increases in the Treg fractions elicited by the tumors. Significant increases in total and CD4+ T cell counts were also observed in individual mice treated with anti-CTLA-4 when compared to mean counts in the untreated group, suggesting a mechanism independent of tumor destruction. Furthermore, CTLA-4 blockade restored CD4+ T cell proliferative capacity and enhanced antitumor immune responses. Interestingly, the benefits of CTLA-4 blockade appear to be bestowed exclusively upon activated CD4+/CD25- T cells but not the Tregs. In the study described above, the CD4+/CD25- T cells obtained from treated mice demonstrated both improved proliferative responses and Treg resistance, whereas Tregs obtained from the same mice remained anergic in vitro and exhibited no restriction of their suppressive effect on effector T cells not treated with CTLA-4 monoclonal antibodies. This absence of a direct effect of anti-CTLA-4 antibodies on Tregs strengthens the notion that CTLA-4 blockade may be synergistic with strategies designed to remove Tregs [89]. Although eliminating suppression of endogenous antitumor immune responses through the removal of Tregs may enhance tumor immune clearance, it is accompanied by a potential risk of inducing autoimmunity, although investigators did not find this in murine models. Strategies that induce Th17 responses but not necessarily Treg inhibition likely are the primary mechanisms of inducing CNS autoimmunity [91]. Nevertheless, CTLA-4 blockade has demonstrated safety and significant efficacy as an antitumor strategy in a variety of animal models [92-95].
The greatest overall clinical experience of Treg modulation in cancer patients is with antibodies that abrogate the function of CTLA-4, including some clinical studies that have included patients with metastatic brain tumors [96,97]. These fully human antibodies (produced by Pfizer [New York, NY] and Bristol-Meyers Squibb [New York, NY]/Medarex [Princeton, NJ]) were created using strains of mice with engineered human immune systems. Their use in clinical trials, mostly for melanoma and prostate cancer [98,99], has been associated with a spectrum of autoimmune-associated side effects, such as dermatitis, enterocolitis, hepatitis, uveitis, and hypophysitis [100], which, in some cases, are associated with clinical response and sustainable progression-free survival [97,101]. Specifically, in one study in which an anti-CTLA-4 antibody was administered with gp100 melanoma-associated antigens to patients with melanoma, 36% of patients with at least grade 3 autoimmune toxic effects had a clinical response, whereas only 5% of those with no autoimmune toxic effects had a clinical response (overall response was 13% regardless of autoimmune toxicity) [102]. This CTLA-4 blockade has been shown to enhance both tumor-specific humoral and cytotoxic responses in patients [103]. Another study found that anti-CTLA-4 immunotherapy with IL-2 in a phase I/II clinical trial in patients with melanoma had an objective response rate of 22% [104]. Interestingly, anti-CTLA-4 therapy has generated clinically meaningful antitumor immunity without autoimmune toxic effects in patients with melanoma or ovarian cancer who were previously vaccinated with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor (GVAX) [105], suggesting that anti-CTLA-4 therapy may be beneficial in previously vaccinated patients. Unfortunately, anti-CTLA-4 therapy has yet to be studied in patients with malignant glioma, but given the aforementioned data, it may be appropriate for use in those glioma patients who have previously received vaccination or immune therapeutics who may be in early stages of progression to provide an immunological “boost.” Furthermore, we propose that the therapeutic efficacy of epidermal growth factor receptor variant III-targeted vaccination [106,107] may be enhanced in combination with CTLA-4 blockade in patients with GBM who have residual disease through reversal of Treg-mediated immunosuppression and may enhance vaccine-induced and endogenous antitumor immune responses.
In the case of anti-CD25, two different immunotoxins have been created that could be exploited for inhibiting Tregs; one is linked to ricin A (RFT5.SMPT-DGA) [108,109], whereas the other is linked to Pseudomonas exotoxin (LMB-2) [110]. Investigators have administered these two immunotoxins in patients with heavily pretreated refractory Hodgkin disease and hairy cell leukemia, respectively, and observed clinical responses. The toxic effects of RFT5.SMPT-DGA included weakness, edema, dyspnea, and myalgia [111], whereas those of LMB-2 consisted primarily of transaminase-level elevations and fever [112]. As with the anti-CTLA-4 approaches, neither of these agents or the other anti-CD25 preclinical approaches that have been investigated in murine models [29,53,86] have been attempted in glioma patients but these agents could also be used in combination with other immune therapeutics or in the setting of residual disease.
In a study of patients with metastatic renal carcinoma, treatment with Ontak (anti-IL-2) specifically depleted Tregs without inducing toxic effects and significantly improved stimulation of tumor-specific T cell responses when those patients were stimulated with tumor RNA-transfected DCs [113]. Without tumor vaccination, researchers have shown that Ontak is efficacious as a single agent against relapsed/refractory B-cell non-Hodgkin lymphoma and cutaneous T cell lymphoma [114]. However, Ontak has not been particularly effective in depleting Tregs in patients with melanoma [61], a tumor of similar neuroectodermal derivation as glioma. Furthermore, Ontak targets IL-2, which is expressed in effector T cells; because the effector T cell immune population is already compromised in patients with GBM [115-117], further suppression of the antitumor effector T cell population may be deleterious in these patients.
Authors have reported that targeting of the Toll-like receptor (TLR) elicits effective antitumor responses against various neoplasms, including experimental brain tumors [118], which may partially neutralize the effects of Tregs. For instance, one study has shown that the stimulation with synthetic or natural ligands for human TLR8, and the subsequent transfer of TLR-8 ligand-stimulated Tregs into tumor-bearing mice led to the enhancement of anti-tumor immunity [119]. Furthermore, a study showed that TLR stimulation by CpG-oligodeoxynucleotides in murine GL261 gliomas enhanced CD8+ T cell mediated immune responses and demonstrated a marked increase in the ratio of CD4+ effector T cells CD4+/Foxp3+ Tregs [120]. However, TLR agonists may not be able to reverse the other mechanisms of immunosuppression in patients with glioma, including immune inhibitory microglia/macrophages present in the tumor microenvironment [50].
Future directions
An emerging approach to modulating Tregs is administration of agents that block or disrupt Notch signaling and STAT-3 inhibitors. Notch plays a role in regulating the responses of T cells and can induce the differentiation of CD4+ T cells into Tregs [121]. However, which member(s) of the Notch family that must be blocked is unclear. As described previously [64,65], the STAT-3 inhibitors are a new class of compounds that are potent inhibitors of Tregs that will soon be tested in clinical trials. Researchers have shown that STAT-3 activation is required for both TGF-β and IL-10 production by CD4+ T cells [122]; both of these factors are necessary for the generation of tumor-associated Tregs. In addition, a study has shown that IL-2 regulates Foxp3 expression in human CD4+/CD25+ Tregs by via STAT-3 binding of the first intron of the Foxp3 gene [123]. Previous studies in mice showed that ablation of Stat3 in the hematopoietic system using the Mx1-Cre-loxP system was accompanied by a reduction in the number of tumor-infiltrating Tregs [124]. We have reported that a small molecular inhibitor of the STAT-3 pathway, WP1066, inhibited the induction of Foxp3 expression in peripheral T cells and downregulated Foxp3 expression in nTregs [64,65] and that this likely accounted for the marked in vivo antitumor effects and enhancement of cytotoxic T cell responses. Additionally, we reported that STAT-3 blockade had negligible inhibitory effects on effector T cell cytotoxicity (which is mediated by the STAT-5 activation pathway), which may compromise immunological tumor clearance. In comparison, many of the other types of anti-Treg agents (Ontak, anti-CD25 antibodies, CTLA-4--blocking agents, etc.) are cross-reactive with other T cell populations that exert effector responses.
Despite recent advancements in immunotherapies for malignant gliomas, the overall prognosis for individuals with these tumors remains very poor. Given the cellular complexity of this very aggressive tumor, any one treatment modality alone is unlikely to be effective against it. Ultimately, improving outcomes in patients with malignant gliomas will require a multifaceted treatment approach combining various treatment modalities. Given the multiplicity of immunosuppressive mechanisms, anti-Treg agents may be most beneficial in patients whose immunosuppression is dependent on this mechanism (i.e., elevated number or fraction of CD4+/Foxp3+ Tregs in the peripheral blood and/or marked intratumoral infiltration with Tregs). Future studies of Treg inhibition/modulation in combination with other immunotherapeutics, such as IFN-α, IL-2, granulocyte-macrophage colony-stimulating factor (GM-CSF), monoclonal antibodies against tumor antigens, peptides, DCs, and anticancer vaccines, would present an opportunity to further potentiate these agents’ therapeutic efficacy, and not just for malignant gliomas. The recent success of several clinical trials of immunotherapy for glioma patients further stresses the need for development of promising adjunct treatments, especially combination treatments, of malignant gliomas. However, these and other future developments must be integrated into a comprehensive treatment program incorporating current treatment modalities.
Acknowledgments
This work was supported by The Dr. Marnie Rose Foundation, the Anthony D. Bullock III Foundation, an institutional research grant from The University of Texas M. D. Anderson Cancer Center, and National Institutes of Health grants CA120813-01 and A177225-01 (to A.B.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Yi-qun Z, Lorre K, de Boer M, et al. B7-blocking agents, alone or in combination with cyclosporin A, induce antigen-specific anergy of human memory T cells. J Immunol. 1997;158:4734–40. [PubMed] [Google Scholar]
- [2].Lim HW, Hillsamer P, Banham AH, et al. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–3. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- [3].Zhao DM, Thornton AM, DiPaolo RJ, et al. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–32. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- [5].Misra N, Bayry J, Lacroix-Desmazes S, et al. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–80. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- [6].Taams LS, van Amelsfort JM, Tiemessen MM, et al. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–30. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Azuma T, Takahashi T, Kunisato A, et al. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63:4516–20. [PubMed] [Google Scholar]
- [8].Ralainirina N, Poli A, Michel T, et al. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol. 2007;81:144–53. doi: 10.1189/jlb.0606409. [DOI] [PubMed] [Google Scholar]
- [9].Smyth MJ, Teng MW, Swann J, et al. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–7. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- [10].Dieckmann D, Plottner H, Berchtold S, et al. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- [12].Jonuleit H, Schmitt E, Stassen M, et al. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Khattri R, Cox T, Yasayko SA, et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- [14].Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- [16].Asano M, Toda M, Sakaguchi N, et al. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bagavant H, Thompson C, Ohno K, et al. Differential effect of neonatal thymectomy on systemic and organ-specific autoimmune disease. Int Immunol. 2002;14:1397–406. doi: 10.1093/intimm/dxf105. [DOI] [PubMed] [Google Scholar]
- [18].Green DR, Webb DR. Saying the ‘S’ word in public. Immunol Today. 1993;14:523–5. doi: 10.1016/0167-5699(93)90180-S. [DOI] [PubMed] [Google Scholar]
- [19].Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- [20].Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- [21].Seddon B, Mason D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor beta and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4(+)CD45RC-cells and CD4(+)CD8(-) thymocytes. J Exp Med. 1999;189:279–88. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25- subpopulations. J Immunol. 2000;165:3105–10. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- [23].Taguchi O, Kontani K, Ikeda H, et al. Tissue-specific suppressor T cells involved in self-tolerance are activated extrathymically by self-antigens. Immunology. 1994;82:365–9. [PMC free article] [PubMed] [Google Scholar]
- [24].Taguchi O, Nishizuka Y. Self tolerance and localized autoimmunity. Mouse models of autoimmune disease that suggest tissue-specific suppressor T cells are involved in self tolerance. J Exp Med. 1987;165:146–56. doi: 10.1084/jem.165.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- [26].Somasundaram R, Jacob L, Swoboda R, et al. Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res. 2002;62:5267–72. [PubMed] [Google Scholar]
- [27].Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- [28].Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13:2158–67. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- [29].Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12:4294–305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- [30].Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–14. [PMC free article] [PubMed] [Google Scholar]
- [31].Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother. 2007;56:271–85. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- [33].Mitchell DA, Fecci PE, Sampson JH. Immunotherapy of malignant brain tumors. Immunol Rev. 2008;222:70–100. doi: 10.1111/j.1600-065X.2008.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323–7. doi: 10.4049/jimmunol.171.12.6323. [DOI] [PubMed] [Google Scholar]
- [35].Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- [37].Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- [38].Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6:745–51. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- [39].Kretschmer K, Apostolou I, Hawiger D, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- [40].Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Scotta C, Soligo M, Camperio C, et al. FOXP3 induced by CD28/B7 interaction regulates CD25 and anergic phenotype in human CD4+CD25- T lymphocytes. J Immunol. 2008;181:1025–33. doi: 10.4049/jimmunol.181.2.1025. [DOI] [PubMed] [Google Scholar]
- [42].Groux H. Type 1 T-regulatory cells: their role in the control of immune responses. Transplantation. 2003;75:8S–12S. doi: 10.1097/01.TP.0000067944.90241.BD. [DOI] [PubMed] [Google Scholar]
- [43].Chen Y, Kuchroo VK, Inobe J, et al. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- [44].Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- [45].Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- [46].Wolf AM, Wolf D, Steurer M, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–12. [PubMed] [Google Scholar]
- [47].Dix AR, Brooks WH, Roszman TL, et al. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216–32. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- [48].Morford LA, Elliott LH, Carlson SL, et al. T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J Immunol. 1997;159:4415–25. [PubMed] [Google Scholar]
- [49].Roszman TL, Brooks WH. Immunobiology of primary intracranial tumours. III. Demonstration of a qualitative lymphocyte abnormality in patients with primary brain tumours. Clin Exp Immunol. 1980;39:395–402. [PMC free article] [PubMed] [Google Scholar]
- [50].Hussain SF, Yang D, Suki D, et al. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–79. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–43. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jordan JT, Sun WH, Hussain SF, et al. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123–31. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Grauer OM, Nierkens S, Bennink E, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121:95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- [54].Akasaki Y, Liu G, Chung NH, et al. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J Immunol. 2004;173:4352–9. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- [55].Aldape K, Ballman K, Furth A, et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63:700–7. doi: 10.1093/jnen/63.7.700. [DOI] [PubMed] [Google Scholar]
- [56].Alvarez JV, Greulich H, Sellers WR, et al. Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res. 2006;66:3162–8. doi: 10.1158/0008-5472.CAN-05-3757. [DOI] [PubMed] [Google Scholar]
- [57].Anichini A, Mortarini R, Maccalli C, et al. Cytotoxic T cells directed to tumor antigens not expressed on normal melanocytes dominate HLA-A2.1-restricted immune repertoire to melanoma. J Immunol. 1996;156:208–17. [PubMed] [Google Scholar]
- [58].Anthony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Aquino A, Prete SP, Greiner JW, et al. Effect of the combined treatment with 5-fluorouracil, gamma-interferon or folinic acid on carcinoembryonic antigen expression in colon cancer cells. Clin Cancer Res. 1998;4:2473–81. [PubMed] [Google Scholar]
- [60].Heimberger AB, Reina-Ortiz C, Yang DS, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–72. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- [61].Attia P, Maker AV, Haworth LR, et al. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–92. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Foss F. Clinical Experience With Denileukin Diftitox (ONTAK) Semin Oncol. 2006;33:11–6. doi: 10.1053/j.seminoncol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- [63].North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kong L-K, Wei J, Sharma AK, et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol Immunother. 2008 doi: 10.1007/s00262-008-0618-y. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kong LY, Abou-Ghazal MK, Wei J, et al. A novel inhibitor of STAT3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008;14:5759–68. doi: 10.1158/1078-0432.CCR-08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Heimberger AB, Sun W, Hussain SF, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cheever MA, Greenberg PD, Fefer A. Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J Immunol. 1980;125:711–4. [PubMed] [Google Scholar]
- [68].Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kedl RM, Rees WA, Hildeman DA, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–13. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Taieb J, Chaput N, Schartz N, et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–9. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- [73].Ercolini AM, Ladle BH, Manning EA, et al. Recruitment of latent pools of high-avidity CD8+ T cells to the antitumor immune response. J Exp Med. 2005;201:1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tsung K, Meko JB, Tsung YL, et al. Immune response against large tumors eradicated by treatment with cyclophosphamide and IL-12. J Immunol. 1998;160:1369–77. [PubMed] [Google Scholar]
- [75].Hengst JCD, Mokyr MB, Dray S. Importance of timing in cyclophosphamide therapy in MOPC-315 tumor-bearing mice. Cancer Res. 1980;40:2135–41. [PubMed] [Google Scholar]
- [76].Hengst JCD, Mokyr MB, Dray S. Cooperation between cyclophosphamide tumoricidal activity and host antitumor immunity in the cure of mice bearing large MOPC-315 tumors. Cancer Res. 1981;41:2163–7. [PubMed] [Google Scholar]
- [77].Holtl L, Ramoner R, Zelle-Rieser C, et al. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother. 2005;54:663–70. doi: 10.1007/s00262-004-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].MacLean GD, Miles DW, Rubens RD, et al. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J Immunother Emphasis Tumor Immunol. 1996;19:309–16. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- [79].Berd D, Maguire HC, McCue P, et al. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunological results in 64 patients. J Clin Oncol. 1990;8:1858–67. doi: 10.1200/JCO.1990.8.11.1858. [DOI] [PubMed] [Google Scholar]
- [80].Plautz GE, Miller DW, Barnett GH, et al. T cell adoptive immunotherapy of newly diagnosed gliomas. Clin Cancer Res. 2000;6:2209–18. [PubMed] [Google Scholar]
- [81].Hermans IF, Chong TW, Palmowski MJ, et al. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63:8408–13. [PubMed] [Google Scholar]
- [82].Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kanzawa T, Germano IM, Komata T, et al. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–57. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- [84].Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–6. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- [85].Sampson JH, Archer GE, Bigner DD, et al. Effect of EGFRvIII-targeted vaccine (CDX-110) induces immune responses and prolongs TTP when given with simultaneous standard and continuous temozolomide in patients with GBM. J Clin Oncol. 2008;26(suppl):92s. [Google Scholar]
- [86].El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006;105:430–7. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- [87].Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Eggena MP, Walker LS, Nagabhushanam V, et al. Cooperative roles of CTLA-4 and regulatory T cells in tolerance to an islet cell antigen. J Exp Med. 2004;199:1725–30. doi: 10.1084/jem.20040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sampson JH, Ashley DM, Archer GE, et al. Characterization of a spontaneous murine astrocytoma and abrogation of its tumorigenicity by cytokine secretion. Neurosurgery. 1997;41:1365–72. doi: 10.1097/00006123-199712000-00024. discussion 72-3. [DOI] [PubMed] [Google Scholar]
- [91].Oukka M. Interplay between pathogenic Th17 and regulatory T cells. Ann Rheum Dis. 2007;66(Suppl 3):iii87–90. doi: 10.1136/ard.2007.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- [93].Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–93. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- [94].Sotomayor EM, Borrello I, Tubb E, et al. In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci U S A. 1999;96:11476–81. doi: 10.1073/pnas.96.20.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yang YF, Zou JP, Mu J, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036–41. [PubMed] [Google Scholar]
- [96].Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–5. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- [99].Theoret MR, Arlen PM, Pazdur M, et al. Phase I trial of an enhanced prostate-specific antigen-based vaccine and anti-CTLA-4 antibody in patients with metastatic androgen-independent prostate cancer. Clin Genitourin Cancer. 2007;5:347–50. doi: 10.3816/CGC.2007.n.017. [DOI] [PubMed] [Google Scholar]
- [100].Blansfield JA, Beck KE, Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–8. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Reuben JM, Lee BN, Shen DY, et al. Therapy with human monoclonal anti-CTLA-4 antibody, CP-675,206, reduces regulatory T cells and IL-10 production in patients with advanced malignant melanoma; 2005 American Society of Clinical Oncology Annual Meeting; Orlando, FL. abstract 7505. [Google Scholar]
- [102].Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yuan J, Gnjatic S, Li H, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–5. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–16. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Heimberger AB, Hussain SF, Aldape K, et al. Tumor-specific peptide vaccination in newly-diagnosed patients with GBM. J Clin Oncol. 2006;24 Part 1. [Google Scholar]
- [107].Sampson JH, Aldape KD, Gilbert MR, et al. Temozolomide as a vaccine adjuvant in GBM. J Clin Oncol. 2007;(Suppl 25):18S. [Google Scholar]
- [108].Engert A, Diehl V, Schnell R, et al. A phase-I study of an anti-CD25 ricin A-chain immunotoxin (RFT5-SMPT-dgA) in patients with refractory Hodgkin’s lymphoma. Blood. 1997;89:403–10. [PubMed] [Google Scholar]
- [109].Schnell R, Vitetta E, Schindler J, et al. Clinical trials with an anti-CD25 ricin A-chain experimental and immunotoxin (RFT5-SMPT-dgA) in Hodgkin’s lymphoma. Leuk Lymphoma. 1998;30:525–37. doi: 10.3109/10428199809057565. [DOI] [PubMed] [Google Scholar]
- [110].Kreitman RJ, Wilson WH, Robbins D, et al. Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood. 1999;94:3340–8. [PubMed] [Google Scholar]
- [111].Schnell R, Vitetta E, Schindler J, et al. Treatment of refractory Hodgkin’s lymphoma patients with an anti-CD25 ricin A-chain immunotoxin. Leukemia. 2000;14:129–35. doi: 10.1038/sj.leu.2401626. [DOI] [PubMed] [Google Scholar]
- [112].Kreitman RJ, Wilson WH, White JD, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–36. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- [113].Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Dang NH, Hagemeister FB, Pro B, et al. Phase II study of denileukin diftitox for relapsed/refractory B-Cell non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:4095–102. doi: 10.1200/JCO.2004.03.071. [DOI] [PubMed] [Google Scholar]
- [115].Miescher S, Whiteside TL, de Tribolet N, et al. In situ characterization, clonogenic potential, and antitumor cytolytic activity of T lymphocytes infiltrating human brain cancers. J Neurosurg. 1988;68:438–48. doi: 10.3171/jns.1988.68.3.0438. [DOI] [PubMed] [Google Scholar]
- [116].Fontana A, Hengartner H, de Tribolet N, et al. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984;132:1837–44. [PubMed] [Google Scholar]
- [117].Roszman TL, Brooks WH, Elliott LH. Inhibition of lymphocyte responsiveness by a glial tumor cell-derived suppressive factor. J Neurosurg. 1987;67:874–9. doi: 10.3171/jns.1987.67.6.0874. [DOI] [PubMed] [Google Scholar]
- [118].Meng Y, Carpentier AF, Chen L, et al. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int J Cancer. 2005;116:992–7. doi: 10.1002/ijc.21131. [DOI] [PubMed] [Google Scholar]
- [119].Peng G, Guo Z, Kiniwa Y, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- [120].Grauer OM, Molling JW, Bennink E, et al. TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol. 2008;181:6720–9. doi: 10.4049/jimmunol.181.10.6720. [DOI] [PubMed] [Google Scholar]
- [121].Hoyne GF, Le Roux I, Corsin-Jimenez M, et al. Serrate1-induced notch signalling regulates the decision between immunity and tolerance made by peripheral CD4(+) T cells. Int Immunol. 2000;12:177–85. doi: 10.1093/intimm/12.2.177. [DOI] [PubMed] [Google Scholar]
- [122].Kinjyo I, Inoue H, Hamano S, et al. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J Exp Med. 2006;203:1021–31. doi: 10.1084/jem.20052333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]