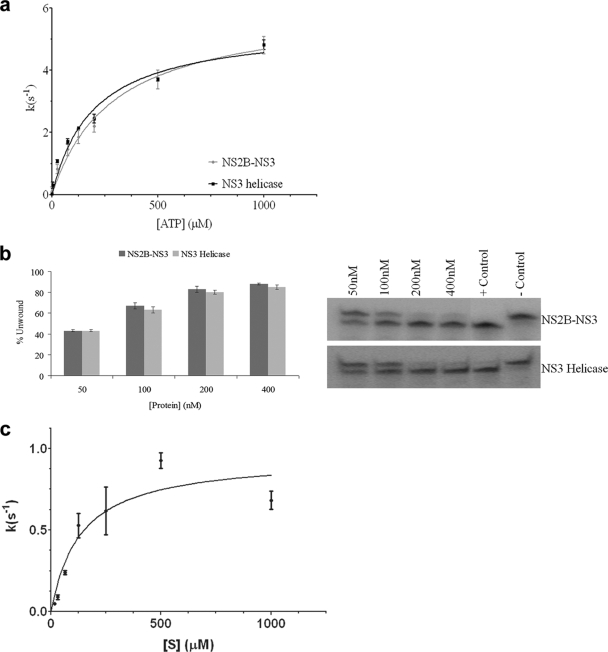
FIG. 2.
Catalytic activity studies of MVEV NS2B45NS3. (a) Comparison of the ATPase activities of MVEV NS2B45NS3 (light gray) and MVEV NS3171-618 (dark gray). The ATPase assay was carried out with 5 nM of enzyme in the presence of the indicated concentrations of ATP. The amount of inorganic phosphate released during catalysis was measured with malachite green. (b) Helicase activities of MVEV NS2B45NS3 (dark gray) and MVEV NS3171-618 (light gray). Unwinding activity was measured by using a radiolabeled double-stranded RNA substrate. Control lanes are included (positive control [heat denatured duplex] and negative control [in the absence of enzyme]). Enzyme concentrations are indicated. The values represent average data from three experiments. (c) Assay of the protease activity of NS2B45NS3 was carried out with 5 nM of enzyme in the presence of the indicated concentrations of peptide (see Materials and Methods). The amount of AMC released during proteolysis was detected by excitation at 354 nm and emission at 442 nm using a SpectraFluorPlus reader (Tecan).