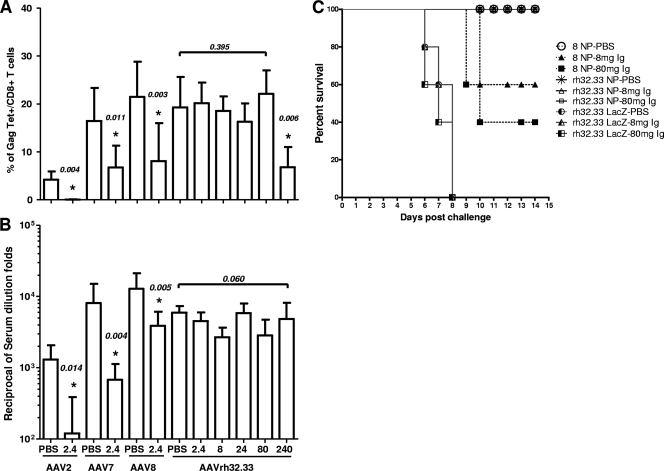
FIG. 5.
Vaccine efficacy in the presence of pooled human Ig. Mice received either PBS or the indicated doses (mg) of pooled human Ig by intravenous administration prior to immunization. Recipient mice were immunized with 3 × 1010 GC of AAV2, -7, -8, or -rh32.33 expressing HIV Gag. The Gag-specific tetramer T-cell responses (A) and Gag-specific antibody responses (B) were measured at 3 weeks postimmunization in the presence or absence of pooled human Ig. The data from 8 to 10 mice in each group are shown as the mean results with standard deviations. Statistically significant differences (P < 0.05) between the IgG-treated vector group and PBS control group are marked with an asterisk. (C) Mice passively transferred with PBS or pooled human Ig (8 or 80 mg) were immunized with 1 × 1011 GC of AAV8 or AAVrh32.33 expressing influenza virus type A NP or AAVrh32.33 expressing LacZ as the control. At day 35 postimmunization, all mice were challenged with 10 50% lethal doses of influenza virus strain PR8. The survival data analysis was plotted using GraphPad Prism (version 5.00 for Windows).