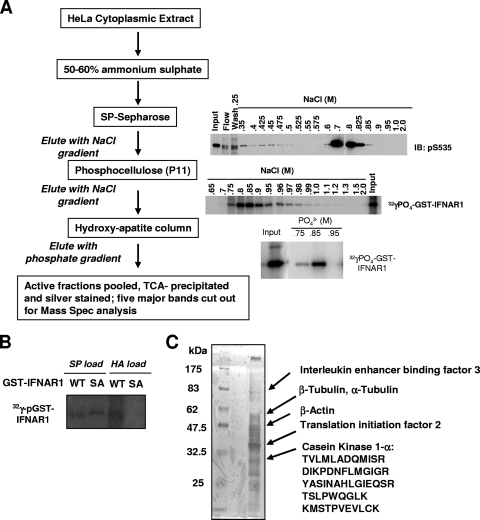
FIG. 1.
Purification of cellular Ser535 kinase activity. (A) Purification scheme and results from in vitro kinase activity assays that used immunoblotting with phospho-specific antibody or [γ-32P]ATP incorporation into the GST-IFNAR1 substrate as indicated. (B) Phosphorylation of bacterium-produced GST-IFNAR1 (wild type or Ser535,539Ala mutant [SA]) by the starting fractions before loading onto either SP Sepharose (SP) or hydroxyappatite (HA) columns in the presence of radioactive [γ-32P]ATP was analyzed by SDS-PAGE and autoradiography. Mutant IFNAR1 migrates slower due to the presence of additional amino acids in the linker between GST and the cytoplasmic domain of IFNAR1 (as outlined in references 31 and 32). (C) Active fractions were pooled after the last purification step. Proteins were precipitated and separated on an SDS-PAGE gel followed by silver staining. Five indicated major bands were cut out for mass spectrometry analysis. The identities of the bands and the sequences of identified CK1α-derived peptides are shown on the right.