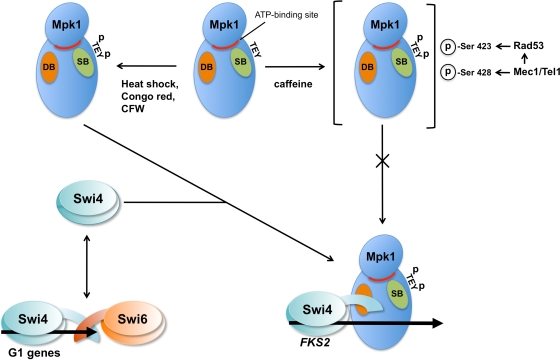
FIG. 10.
Model for interaction of Mpk1 with Swi4. Mpk1 activated by cell wall stressors other than caffeine is capable of activating Swi4-dependent transcription. Swi4 binds Mpk1 through the D motif near the C terminus of Swi4, which allows Swi4 to bind the FKS2 promoter. This interaction is proposed to relieve the autoinhibitory interaction of the Swi4 C terminus that prevents DNA binding. In this regard, Mpk1 serves the same function as Swi6 in allowing Swi4 to bind DNA. Swi6 is recruited to the Mpk1-Swi4 complex and binds to Swi4 through their respective C termini (not shown), in a manner similar to their interaction on G1-induced cell cycle genes (e.g., CLN2). Mpk1 activated by caffeine is not capable of Swi4-driven transcription. Under these conditions, activated Mpk1 is incapable of interacting with Swi4. Additional phosphorylations on Mpk1 Ser423 and Ser428 are provoked by caffeine treatment through the DNA damage checkpoint kinases Mec1/Tel1 and Rad53. Phosphorylation of Ser423, which is dependent on Rad53, specifically prevents Mpk1 binding to Swi4. DB, D-motif-binding site; SB, substrate-binding site; p, phosphate group; CFW, calcofluor white.