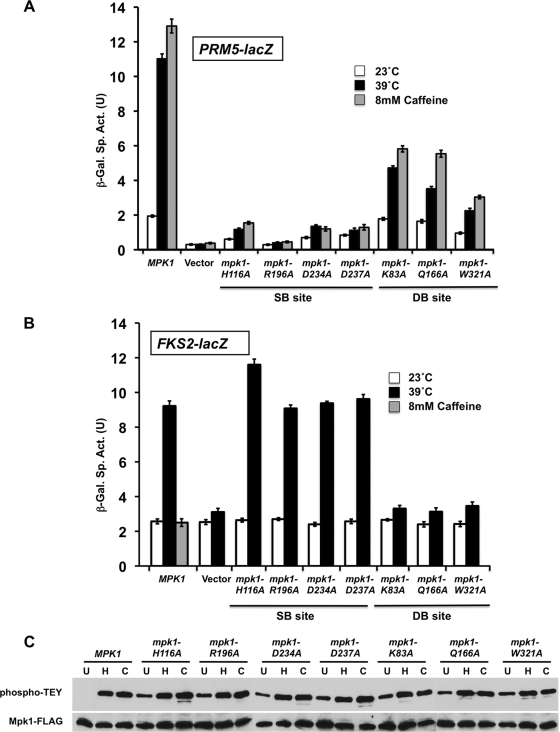
FIG. 2.
Mutations in the substrate-binding site and the D-motif-binding site of Mpk1 cause differential effects on cell wall stress induction of FKS2 and PRM5 expression. (A) Cell wall stress-induced PRM5 transcription. A PRM5-lacZ reporter plasmid (p1366) was cotransformed with centromeric plasmids expressing MPK1-FLAG (p2704), plasmids with the indicated point mutants in MPK1, or empty vector (pRS315) into an mpk1Δ mutant (yeast strain DL3195). Transformants were grown to saturation at 23°C in SD medium with 10% sorbitol lacking Ura and Leu. Cultures were diluted into 100 ml of medium so that subsequent incubation at 23°C, 39°C, or 23°C with 8 mM caffeine for 2 h resulted in mid-log-phase cultures (A600 of 1.0). β-Galactosidase activity was measured in crude extracts. The specific activity of β-galactosidase (in units) [β-Gal Sp. Act. (U)] is shown on the y axis. Each value represents the mean ± standard deviation (error bar) from three independent transformants. (B) Cell wall stress-induced FKS2 transcription. An FKS2-lacZ reporter plasmid (p2052) was cotransformed with centromeric plasmids expressing MPK1 (p2704), plasmids with the indicated point mutations in MPK1, or empty vector (pRS315) into an mpk1Δ mlp1Δ mutant (DL3196). The mpk1Δ mlp1Δ mutant was used for this experiment because the Mlp1 pseudokinase contributes to FKS2 expression. Transformants were grown as described above and diluted into 100 ml of medium so that subsequent incubation at 23°C, 39°C, or 23°C with 8 mM caffeine (MPK1 only) for 15 h resulted in mid-log-phase cultures (A600 of 1.0 to 1.5). (C) Point mutations in Mpk1 are not compromised for phosphorylation by their activating protein kinases (Mkk1/2). Protein extracts from cells treated as in panel A (left untreated at 23°C [lanes U], heated to 39°C [lanes H], or treated with 8 mM caffeine [lanes C]) were subjected to immunoprecipitation of Mpk1-FLAG with anti-FLAG antibodies followed by SDS-PAGE. Dual phosphorylation of Mpk1 was detected with anti-phospho-p44/p42 MAPK antibodies; total Mpk1 was detected with anti-FLAG antibodies.