Abstract
Peritoneal dialysis (PD) is a well-established form of therapy for stage 5 chronic kidney disease requiring renal replacement therapy. d-Glucose has been used successfully for several decades as the osmotic agent employed in dialysis solutions to achieve adequate fluid removal. The absorption of 100–200 grams of glucose per day has been suggested as potentially increasing cardiometabolic risk, particularly in patients with diabetes. Supporting and undermining evidence for this hypothesis is reviewed, with a focus on the role of glucose absorption in changes in body composition, dyslipidemia, and glycemic control in diabetic PD patients. Clinical strategies to optimize fluid removal while minimizing the metabolic impact of glucose absorption are also discussed.
Keywords: cardiometabolic, glucose, icodextrin, peritoneal dialysis
Introduction
Peritoneal dialysis (PD) is a well-established form ofrenal replacement therapy for patients with stage 5 chronic kidney disease, having grown from its inception in the 1970s to today, where it is used by over 170,000 individuals globally.1 The annual global growth rate of this therapy is between 6 and 7%, whereas developing regions, especially Asia, are witnessing growth rates of up to 25% or greater. In the United States, according to the 2008 United States Renal Data System report, of the 26,082 PD patients, 33.7% were classified as having diabetes.1 For several decades since the 1970s, PD solutions relied mainly on d-glucose as the osmotic agent of choice. d-Glucose is a biologically active stereoisomer, often referred to as dextrose. Glucose offers a good osmotic driving force, resulting in rapid ultrafiltration (UF), especially in the first few hours of intraperitoneal dwell. In addition, the metabolic fate of glucose is well understood, with no acute toxicity or proven long-term side effects. Glucose can be heat sterilized, permitting a large margin of safety in terms of sterility assurance of the commercial product. Glucose is also stable for long periods of time in dialysis solution formulations at ambient temperature ranges, thereby permitting an acceptable shelf life.
In recent years, the attractive attributes of glucose as an osmotic agent in PD have been tempered by growing speculation on the potential clinical disadvantages of excessive glucose exposure, especially where daily fluid removal needs require the use of high glucose concentration solutions. Depending on their peritoneal membrane transporter characteristics, PD patients can absorb from approximately 100 to 200 grams of glucose per day.2 The availability of nonglucose osmotic agents in recent years in many countries around the world has resulted in several, albeit usually small, interventional studies ascribing potential metabolic benefits to nonglucose-based solutions, thus fueling interest in their potential to reduce cardiometabolic risk. The most widely available nonglucose PD solution today is a 7.5% icodextrin solution. Icodextrin is a mixture of high molecular weight glucose polymers isolated from hydrolyzed corn starch. Icodextrin provides a colloidal rather than a crystalloid osmotic force, thereby inducing a slower but more sustained removal of fluid via peritoneal UF and thus is suited ideally for the long dwell in PD. A 1.1% amino acid-based solution that contains no glucose while providing UF equivalent to a 1.5% dextrose monohydrate solution is also available in some countries and is indicated primarily for malnourished patients.
In 2007, Holmes2 provided an overview of glucotoxicity associated with conventional glucose-based PD, including supportive and undermining clinical and epidemiologic evidence available at that time with potential clinical approaches to minimize glucose exposure. These themes are expanded and updated in this review with particular emphasis on the diabetic PD patient.
Proposed Pathways of Glucotoxicity in Diabetic PD Patients
Intraperitoneal instillation of conventional glucose-based PD solutions may result in both local (peritoneal) effects and, because about 50 to 80% of the instilled glucose is absorbed, systemic effects. The putative pathways of both local and systemic glucotoxicity are depicted in Figure 1. These pathways lead to structural and functional changes of the peritoneal membrane, resulting in fluid overload, impaired glucose control, altered lipid profiles, and accumulation of atherogenic visceral fat. This review focuses on the role of systemic glucose absorption in cardiometabolic risk. The thesis that excessive glucose exposure plays an important role in the outcomes of diabetic PD patients remains controversial, as much of the supportive evidence is indirect and adequately powered interventional trials comparing glucose sparing prescriptions to conventional ones are limited. Evidence for and against a role of glucose absorption in cardio-metabolic risk in diabetic PD patients is discussed in the following sections.
Figure 1.
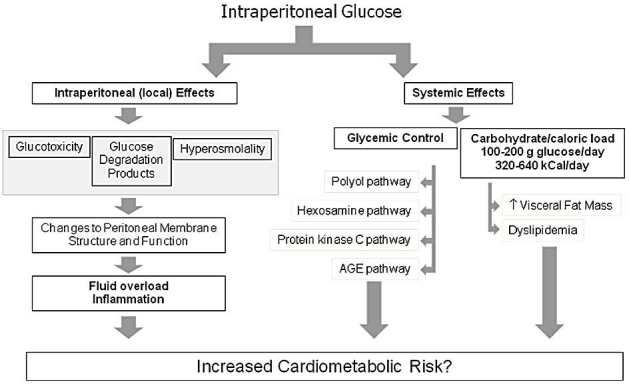
Putative pathways of glucose-associated cardiometabolic risk.
Glycemic Control in Diabetic PD patients
In order to study the effect of intraperitoneal glucose on glycemic control in diabetic patients, Marshall and colleagues3 employed a continuous glucose monitoring system to profile 24-hour blood glucose control in eight insulin-dependent diabetic continuous ambulatory peri-toneal dialysis (CAPD) patients using a crossover design to compare glucose-based PD solutions to a prescription that incorporated both icodextrin and an amino acid-based solution. A significant reduction in mean 24-hour blood glucose in the glucose sparing phase and a significantly tighter control of blood glucose levels were observed (Figure 2), confirming that improved blood glucose control can be achieved with nonglucose-based dialysis solutions. Blood glucose measurements in patients receiving icodextrin must be done with a glucose-specific method to avoid interference by maltose, a metabolite of icodextrin. Glucose dehydrogenase pyrroloquinoline quinone or glucose dye oxidoreductase-based methods must not be used.
Figure 2.
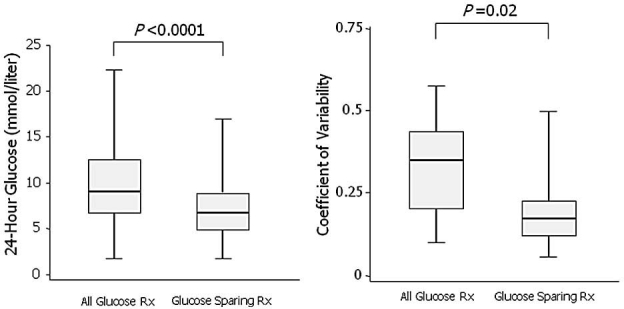
Effect of nonglucose solutions on glucose control with diabetic PD patients. Modified from Marshall and colleagues.3
Early studies evaluating the effect of the nonglucose osmotic agent icodextrin on hemoglobin A1c (HbA1c) have been contradictory, possibly caused by inadequately powered designs.4,5 However, two recent prospective, multicenter open-labeled studies have reported that a significant HbA1c reduction could be achieved with icodextrin use.6,7 The most recent of these was a randomized controlled study of 59 diabetic CAPD patients with a 12-month follow-up period, where HbA1c values rose by 0.79% in the glucose-only group versus a fall of 0.98% in the icodextrin group (p < 0.01).6
Despite observations of improved glycemic control with nonglucose solutions described earlier, controversy remains as to its clinical importance per se, as limited evidence exists over the benefit of glycemic control in general in the diabetic end stage renal disease (ESRD) population, especially in the PD population.8 Two large U.S. national dialysis chain database studies of hemodialysis (HD) patients have gained much attention recently. No association between HbA1c levels and survival was seen at 12 months in a cohort of over 24,000 diabetic patients.9 A second study of equal size found a paradoxical lower unadjusted mortality with greater HbA1c levels, which became associated with a greater mortality after adjustment for markers for malnutrition and inflammation.8 A 1993 report by Tzamaloukas and colleagues10 of 39 type 1 and 71 type 2 patients concluded that poor glycemic control (defined by <50% of blood glucose measurements within 3.3–11.1 mmol/liter) was associated with significantly higher HbA1c values versus a well-controlled control group with higher rates of both microvascular and macrovascular disease and significantly lower rates of technique and patient survival.
Alternative measures to HbA1c for glycemic control assessment in PD patients, such as glycated albumin and skin advanced glycation end product levels, have been described as more accurate predictors of long-term glycemic control in this population.11,12 For example, in 2005, Meerwaldt and associates13 established that the baseline skin advanced glycation end product level, as measured by autofluorescence, independently predicts overall and cardiovascular survival in HD patients. Pertinent to this review, a preliminary report by McIntyre and colleagues14 demonstrated that the skin advanced glycation end product concentration correlates with dialysis vintage in PD patients, unlike HD patients, and is strongly associated with the degree of historical PD glucose exposure. If confirmed, this observation would provide strong evidence for an important role of glucose exposure in both diabetic and nondiabetic PD patients.
Weight Gain, Body Mass Index, Visceral Fat, and Adipokines in PD Patients
The role of glucose absorption in changes in body composition of PD patients remains controversial. It is well established that PD patients frequently gain weight, especially during the first year of PD therapy and particularly if they have diabetes or have a high body mass index (BMI) at initiation. Glucose absorption in PD patients can equal approximately 15 to 30% of the recommended daily calorie intake for an ambulant 80-kg diabetic male, and thus often is assumed to be the cause of weight gain. However, several groups have not found any association between glucose absorption and weight gain.15,16 Although this may appear incongruous upon initial consideration, studies on the role of specific genetic polymorphisms that influence total energy expenditure may provide some explanation.
Wang and colleagues17 have demonstrated eloquently the important role of a UCP2 gene polymorphism in regulating a fat mass increase in PD patients. UCP2 likely plays an important role in regulating energy balance by participating in both resting energy expenditure and fatty acid oxidation. PD patients with the UCP2 del/del genotype (57%) showed a significant increase in total and truncal fat mass in this study (Figure 3). This association was not seen in HD patients, illustrating the important interaction between this polymorphism and some element specific to PD. Furthermore, Vasselai and colleagues16 observed that PD patients who gained body fat did so because of a higher ratio of total energy intake (absorbed glucose plus oral intake) to resting energy expenditure. Consequently, the existence of genetic polymorphic groups within the PD patient population and their influence on total energy balance may explain the prior lack of correlation between the single parameter of glucose uptake and weight gain when studying a mixed polymorphic population.
Figure 3.
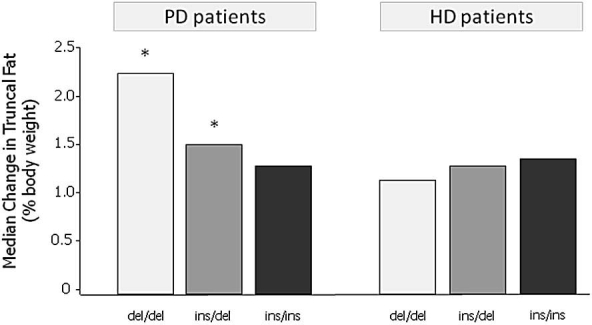
Importance of UCP2 gene polymorphisms in changes in body fat during the first year of dialysis. del, deletion; ins, insertion. Modified from Wang and associates.17
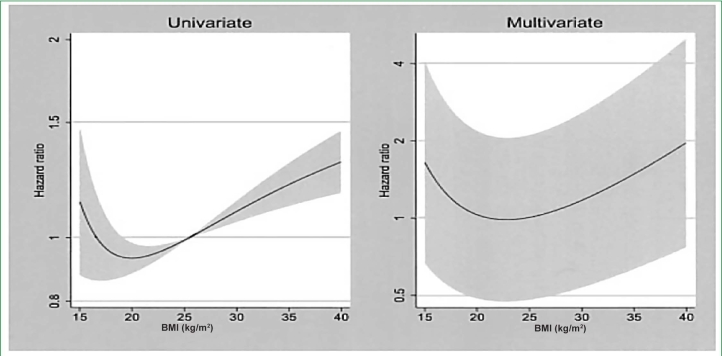
Despite the daily caloric contribution that glucose provides to PD patients, no major differences in BMI distribution have been identified to date between HD and PD patients. Differences do appear to exist between the survival risk posed by an increasing BMI between PD and HD patients. Obesity has been associated with improved survival in HD patients and is often referred to as an example of the reverse epidemiology of chronic kidney disease. In contrast, a detailed review concluded that, at least in the United States, obese PD patients in contrast to HD patients do not appear to have a survival advantage compared to normal BMI patients and, if diabetic, may have an increased risk of death after starting PD as compared to HD.18 Johnson and colleagues5 has highlighted the potential for selection bias in U.S. patient studies due to the relatively low utilization of PD versus HD. A careful analysis of the Australian and New Zealand Dialysis and Transplant Association registry found that obesity, as defined by a BMI >30 kg/m2, was associated independently with death and technique failure in this large cohort of PD patients where selection of PD and HD was more equal.19 Furthermore, when BMI was analyzed as a continuous variable, a J-shaped curve was reported, with greatest survival at a BMI of 20 kg/m2 and survival decreasing progressively with BMI values both below and above that value (Figure 4).
Figure 4.
Univariate and multivariate fractional polynomial graphs defining the relationship between BMI and mortality risk in the Australian/New Zealand PD population. Shaded areas represent 95% confidence limits. Reproduced with permission from Journal of the American Society of Nephrology.
It is becoming apparent that BMI as a summary statistic may not be the optimal measure of body composition for comparison between PD and HD patients. The location of fat accumulation, vis-à-vis abdominal versus peripheral, has different metabolic implications, with abdominal fat being associated with increased cardiovascular risk in both peritoneal and HD patients.20,21 This has ledinvestigators to propose future use of new anthropometric tools that estimate the relative accumulation of abdominal fat, such as the conicity index, rather than relying solely on BMI. In addition, because protein energy wasting (PEW) appears to strongly influence inflammation and mortality in ESRD patients, inclusion of PEW to assess the role of sarcopenia in the presence of high BMI may be important.22
Wang and colleagues17 have also showed that the increase in truncal fat mass observed over time in PD patients was associated positively with serum leptin, interleukin-6, and dyslipidemia and was correlated negatively with adiponectin levels, supporting the hypothesis that increased truncal fat mass may contribute to cardiometabolic risk. These observations support a preceding observational study of 36 PD patients that reported a significant increase in total body mass, truncal fat mass, and serum leptin levels over the first year after commencement of therapy, noting that these changes were most pronounced in patients with diabetes.23 The cause for the large increase in leptin levels after commencement of PD is likely due to the increase in body fat content, as leptin is produced almost exclusively by adipose tissue. Teta and colleagues24 have demonstrated that, in vitro, adipocytes reveal a concentration-dependent secretion of leptin with glucose, which is not seen with nonglucose dialysis solutions such as icodextrin or amino acid solutions. Acute dwell studies in patients also revealed24 that an amino acid-based solution did not elevate plasma leptin concentrations, unlike glucose-based solutions. Two small clinical studies from Japan have evaluated the effect of icodextrin on plasma leptin levels in mainly nondiabetic PD patients with opposite conclusions.25,26 Reasons forthis discrepancy are unclear but likely include differences in study design and statistical power.
Dyslipidemia of PD Patients
The dyslipidemia of PD patients has been well characterized and is reported most often as elevated total cholesterol, low-density lipoprotein (LDL) cholesterol, lipoprotein(a) [Lp(a)], triglyceride, and apolipoprotein B (apoB) levels. Compared to HD patients, PD patients are often ascribed a more atherogenic profile because they exhibit higher LDL cholesterol and apoB levels, along with elevated triglycerides. In 2004, Pennell and colleagues27 clearly demonstrated that switching patients with hyperlipidemia from PD to HD reduced all LDL lipoproteins and triglycerides significantly. A multicenter 12-month observational study by Babazono and associates7 of 51 CAPD diabetic patients using icodextrin for their overnight dwell reported a striking positive correlation between changes in triglyceride levels and glucose absorption (Figure 5), whereas total and LDL cholesterol levels and triglycerides decreased significantly over the observation period. In a randomized controlled trial, Paniagua and colleagues6 also reported decreased tri-glyceride levels in diabetic PD patients treated with icodextrin.
Figure 5.
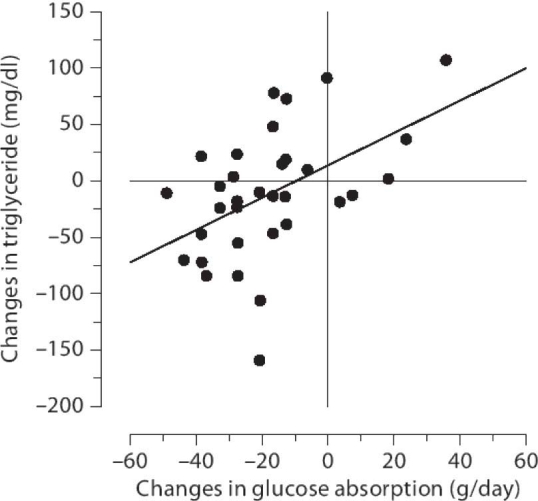
Correlation between changes in triglyceride levels and estimated glucose absorption at 3 months from baseline when treated with icodextrin (r = 0.486, p = 0.004, n = 34). Reproduced with permission from Journal of Nephrology.
Mortality Studies in Dialysis Patients: Are Diabetic PD Patients at Risk?
If glucose absorption does potentially contribute to increased cardiometabolic risk in PD patients, are there any differences in survival between PD and HD patients that would support this hypothesis? In 2006, Vonesh and colleagues28 provided a critical review of six large-scale national registries and three prospective studies and concluded that, after accounting for differences in case mix adjustment, survival on PD was generally found to be equal or better than HD among nondiabetic and younger diabetic patients, whereas the survival of older diabetes patients varied by country. Older diabetic patients (age 45 and older) fared worse than HD patients in the United States, whereas no difference was seen in the Danish and Canadian studies. The observed difference in survival between older diabetic PD and HD patients in the aforementioned studies is unclear, but excessive glucose exposure has been suggested as a contributing factor. More recently, there have been at least three large national database studies comparing PD and HD survival.29–31 These studies do not significantly affect the overall conclusions of the Vonesh review given earlier, as they essentially build upon the scientific equipoise in this area.
It is clear that observational data will unlikely delineate the role that excessive glucose absorption plays in cardio-metabolic risk in diabetic PD patients. Interventional studies using glucose sparing strategies will likely be the definitive method to establish the importance of glucose absorption in cardiometabolic risk. In this respect, it is noted that an adequately powered randomized controlled clinical trial is underway in diabetic CAPD and automated peritoneal dialysis (APD) patients comparing glucose-only prescriptions to glucose sparing ones that employ a combination of icodextrin, amino acids, and a bicarbonate/lactate-buffered biocompatible glucose-based solution.32 The primary end point of this study is HbA1c, with a variety of secondary outcome measures, including glycemic control medication, hypoglycemic events, and quality of life.
Optimizing Dialysis Solution Exposure in Glucose in Peritoneal Dialysis
Clinically, glucose absorption can be minimized either by reducing the need for peritoneal UF or by optimizing the UF efficiency of the dialysis therapy. Reducing the need for peritoneal UF is best achieved with a reduction in dietary salt and water intake and the use of diuretics. Preservation of residual renal function is also a first-line strategy to minimize glucose exposure, and thus the use of angiotensin-converting enzyme/angiotensin II receptor blockers, while avoidance of dye studies, nonsteroidal anti-inflammatory drugs (including cyclooxygenase-2 inhibitors), aminoglycosides, and extracellular fluid depletion, whenever possible, is recommended.
Ultrafiltration efficiency is usually defined as the amount of net UF obtained for every gram of carbohydrate absorbed. Ultrafiltration efficiency is a dynamic parameter that changes over the dwell time because the volume of UF and the amount of the osmotic agent change over time. Ultrafiltration efficiency is also affected by the peritoneal membrane characteristic (slow/low to fast/high absorbers), the concentration of the osmotic agent used, and the volume of the infused solution. Clinically, optimizing UF efficiency with glucose-based solutions by varying dwell times, fill volumes, and various glucose concentrations is impractical without mathematical modeling capabilities, whereas the use of nonglucose-based solutions is a simple, pragmatic approach. The beneficial effect of icodextrin on the UF efficiency of the APD long dwell in a randomized controlled phase III trial is shown in Figure 6. The superior UF efficiency of icodextrin is a product of the superior UF and the lower carbohydrate absorption versus glucose. At first consideration, because only about 12 grams of carbohydrate absorption was saved using icodextrin in this study, a conclusion may be drawn that the glucose sparing benefit is limited. However, it should be appreciated that in order to achieve the same daily fluid removal with a glucose-based only solution prescription as that achieved when using icodextrin, a significant increase in the glucose concentration will be required. This is illustrated in Figure 7 using a mathematical modeling approach. When using icodextrin for a long dwell in an average PD patient, achieving an equivalent daily UF using glucose-only solutions requires a higher number of concentrated glucose solutions, resulting in approximately an additional daily absorption of 40 grams of glucose.
Figure 6.
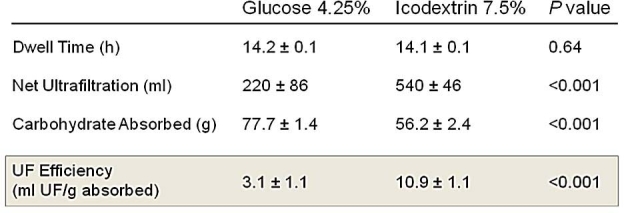
Comparison of ultrafiltration efficiency of 4.25% glucose and 7.5% icodextrin during the long dwell in APD patients. Modified from Holmes.2
Figure 7.
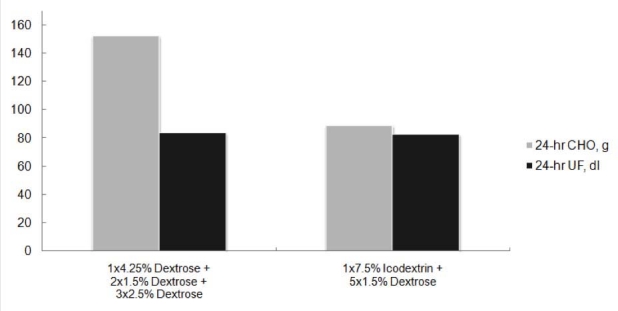
Mathematical modeling construction depicting the impact of icodextrin on daily carbohydrate (CHO) absorption when using prescriptions that achieve equivalent daily fluid removal. Assumption: two liter fill volumes, daytime dwell of 14 hours, and nighttime therapy of five 2-hour dwells. The model is a mix of transporter types representative of the APD population (15% high, 37% high-average, 33% low-average, 15% low).
Future Research Needs
Although an emerging body of evidence suggests that glucose sparing dialysis solutions may reduce cardiometabolic risk factors in diabetic and nondiabetic PD patients, the ability to demonstrate a reduction in mortality and an improvement in quality of life will always be challenging due to the logistical challenges of enrolling adequate numbers of patients for such studies. Consequently, there is a critical need to establish which risk factors are strong predictors of decreased morbidity and mortality and improved quality of life in this population. For instance, is skin advanced glycation end product content, glycated albumin, or fructosamine measurement superior to HbA1c as a measure of glycemic control in PD patients? Similarly, despite much reliance on body mass index as a measure of body composition in dialysis patients to date, are changes in visceral fat mass over time more important to outcomes? Establishment of strong surrogate predictors of meaningful clinical outcomes would facilitate the feasibility of randomized interventional studies of glucose sparing strategies.
Summary
D-Glucose has been used successfully for several decades as the prototypical osmotic agent employed in dialysis solutions to achieve adequate fluid removal. The absorption of 100–200 grams of glucose per day in PD patients has been suggested as potentially increasing cardiometabolic risk, particularly in patients with diabetes. The role that glucose absorption plays in changes in body composition, dyslipidemia, and glycemic control of diabetic PD patients remains to be fully defined. Clinical strategies used to optimize glucose exposure in diabetic PD patients include reduction of dietary salt and water intake, preservation of residual function, use of diuretics, and substitution of glucose-based solutions with nonglucose alternatives, such as icodextrin. Interventional studies with icodextrin suggest that improved glycemic control and an improved lipid profile in diabetic PD patients are possible with new solution formulations, although confirmation via ongoing adequately powered randomized controlled trials is needed.
Acknowledgment
The author acknowledges the assistance of Alp Akonaur, Ph.D. for his mathematical modeling contribution.
Abbreviations
- APD
automated peritoneal dialysis
- apoB
apolipoprotein B
- BMI
body mass index
- CAPD
continuous ambulatory peritoneal dialysis
- ESRD
end stage renal disease
- HbA1c
hemoglobin A1c
- HD
hemodialysis
- LDL
low density lipoprotein
- [Lp(a)]
lipoprotein(a)
- PD
peritoneal dialysis
- PEW
protein energy wasting
- UF
ultrafiltration
References
- 1.United States Renal Data System. USRDS Coordinating Center. 2008. Available from: http://www.usrds.org.
- 2.Holmes CJ. Glucotoxicity in peritoneal dialysis–solutions for the solution! Adv Chronic Kidney Dis. 2007;14(30):269–278. doi: 10.1053/j.ackd.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Marshall J, Jennings P, Scott A, Fluck RJ, McIntyre CW. Glycemic control in diabetic CAPD patients assessed by continuous glucose monitoring system (CGMS) Kidney Int. 2003;64(4):1480–1486. doi: 10.1046/j.1523-1755.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 4.Hithaishi C, Lobbedez T, Padmanabhan S, Pineda ME, Oreopoulos DG. No beneficial effect of icodextrin on blood glucose control. Perit Dial Int. 2004;24(2):199–200. [PubMed] [Google Scholar]
- 5.Johnson DW, Arndt M, O'Shea A, Watt R, Hamilton J, Vincent K. Icodextrin as salvage therapy in peritoneal dialysis patients with refractory fluid overload. BMC Nephrol. 2001;2:2. doi: 10.1186/1471-2369-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paniagua R, Ventura MD, Avila-Diaz M, Cisneros A, Vicente-Martinez M, Furlong MD, Garcia-Gonzalez Z, Villanueva D, Orihuela O, Prado-Uribe MD, Alcantara G, Amato D. Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int. 2009;29(4):422–432. [PubMed] [Google Scholar]
- 7.Babazono T, Nakamoto H, Kasai K, Kuriyama S, Sugimoto T, Nakayama M, Hamada C, Furuya R, Hasegawa H, Kasahara M, Moriishi M, Tomo T, Miyazaki M, Sato M, Yorioka N, Kawaguchi Y. Effects of icodextrin on glycemic and lipid profiles in diabetic patients undergoing peritoneal dialysis. Am J Nephrol. 2007;27(4):409–415. doi: 10.1159/000105123. [DOI] [PubMed] [Google Scholar]
- 8.Kovesdy CP, Sharma K, Kalantar-Zadeh K. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis. 2008;52(4):766–777. doi: 10.1053/j.ajkd.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Williams ME, Lacson E, Jr., Teng M, Ofsthun N, Lazarus JM. Hemodialyzed type I and type II diabetic patients in the US: Characteristics, glycemic control, and survival. Kidney Int. 2006;70(8):1503–1509. doi: 10.1038/sj.ki.5001789. [DOI] [PubMed] [Google Scholar]
- 10.Tzamaloukas AH, Yuan ZY, Murata GH, Balaskas E, Avasthi PS, Oreopoulos DG. Clinical associations of glycemic control in diabetics on CAPD. Adv Perit Dial. 1993;9:291–294. [PubMed] [Google Scholar]
- 11.Meerwaldt R, Zeebregts CJ, Navis G, Hillebrands JL, Lefrandt JD, Smit AJ. Accumulation of advanced glycation end products and chronic complications in ESRD treated by dialysis. Am J Kidney Dis. 2009;53(1):138–150. doi: 10.1053/j.ajkd.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Peacock TP, Shihabi ZK, Bleyer AJ, Dolbare EL, Byers JR, Knovich MA, Calles-Escandon J, Russell GB, Freedman BI. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73(9):1062–1068. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 13.Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, Thorpe SR, Baynes JW, Navis G, Gans RO, Smit AJ. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16(12):3687–3693. doi: 10.1681/ASN.2005020144. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre N, McIntyre CW, John S, Chesterton L, Jeffres H, Korsheed S, Owen P, Burton J, Fluck R. Tissue advanced glycation end product concentration in dialysis patients. Milan, Italy: World Congress of Nephrology; 2009. p. Su416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little J, Phillips L, Russell L, Griffiths A, Russell GI, Davies SJ. Longitudinal lipid profiles on CAPD: their relationship to weight gain, comorbidity, and dialysis factors. J Am Soc Nephrol. 1998;9(10):1931–1939. doi: 10.1681/ASN.V9101931. [DOI] [PubMed] [Google Scholar]
- 16.Vasselai P, Kamimura MA, Bazanelli AP, Pupim LB, Avesani CM, da Mota Ribeiro FS, Manfredi SR, Draibe SA, Cuppari L. Factors associated with body-fat changes in prevalent peritoneal dialysis patients. J Ren Nutr. 2008;18(4):363–369. doi: 10.1053/j.jrn.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Axelsson J, Nordfors L, Qureshi AR, Avesani C, Barany P, Schalling M, Heimburger O, Lindholm B, Stenvinkel P. Changes in fat mass after initiation of maintenance dialysis is influenced by the uncoupling protein 2 exon 8 insertion/deletion polymorphism. Nephrol Dial Transplant. 2007;22(1):196–202. doi: 10.1093/ndt/gfl504. [DOI] [PubMed] [Google Scholar]
- 18.Abbott KC, Oliver DK, Hurst FP, Das NP, Gao SW, Perkins RM. Body mass index and peritoneal dialysis: “exceptions to the exception” in reverse epidemiology? Semin Dial. 2007;20(6):561–565. doi: 10.1111/j.1525-139X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 19.McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol. 2003;14(11):2894–2901. doi: 10.1097/01.asn.0000091587.55159.5f. [DOI] [PubMed] [Google Scholar]
- 20.Lu Q, Cheng LT, Wang T, Wan J, Liao LL, Zeng J, Qin C, Li KJ. Visceral fat, arterial stiffness, and endothelial function in peritoneal dialysis patients. J Ren Nutr. 2008;18(6):495–502. doi: 10.1053/j.jrn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T, Kuno T, Takada H, Nagura Y, Kanmatsuse K, Takahashi S. The impact of visceral fat on multiple risk factors and carotid atherosclerosis in chronic haemodialysis patients. Nephrol Dial Transplant. 2003;18(9):1842–1847. doi: 10.1093/ndt/gfg261. [DOI] [PubMed] [Google Scholar]
- 22.Honda H, Qureshi AR, Axelsson J, Heimburger O, Suliman ME, Barany P, Stenvinkel P, Lindholm B. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007;86(3):633–638. doi: 10.1093/ajcn/86.3.633. [DOI] [PubMed] [Google Scholar]
- 23.Stenvinkel P, Lindholm B, Lonnqvist F, Katzarski K, Heimburger O. Increases in serum leptin levels during peritoneal dialysis are associated with inflammation and a decrease in lean body mass. J Am Soc Nephrol. 2000;11(7):1303–1309. doi: 10.1681/ASN.V1171303. [DOI] [PubMed] [Google Scholar]
- 24.Teta D, Maillard M, Halabi G, Burnier M. The leptin/adiponectin ratio: potential implications for peritoneal dialysis. Kidney Int Suppl. 2008:S112–S118. doi: 10.1038/sj.ki.5002611. [DOI] [PubMed] [Google Scholar]
- 25.Furuya R, Odamaki M, Kumagai H, Hishida A. Beneficial effects of icodextrin on plasma level of adipocytokines in peritoneal dialysis patients. Nephrol Dial Transplant. 2006;21(2):494–498. doi: 10.1093/ndt/gfi197. [DOI] [PubMed] [Google Scholar]
- 26.Takeguchi F, Nakayama M, Nakao T. Effects of icodextrin on insulin resistance and adipocytokine profiles in patients on peritoneal dialysis. Ther Apher Dial. 2008;12(3):243–249. doi: 10.1111/j.1744-9987.2008.00581.x. [DOI] [PubMed] [Google Scholar]
- 27.Pennell P, Rojas C, Asif A, Rossini E. Managing metabolic complications of peritoneal dialysis. Clin Nephrol. 2004;62(1):35–43. doi: 10.5414/cnp62035. [DOI] [PubMed] [Google Scholar]
- 28.Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006:S3–S11. doi: 10.1038/sj.ki.5001910. [DOI] [PubMed] [Google Scholar]
- 29.Liem YS, Wong JB, Hunink MG, de Charro FT, Winkelmayer WC. Comparison of hemodialysis and peritoneal dialysis survival in The Netherlands. Kidney Int. 2007;71(2):153–158. doi: 10.1038/sj.ki.5002014. [DOI] [PubMed] [Google Scholar]
- 30.McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol. 2009;20(1):155–163. doi: 10.1681/ASN.2007111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mircescu G, Garneata L, Florea L, Cepoi V, Capsa D, Covic M, Gherman-Caprioara M, Gluhovschi G, Golea OS, Barbulescu C, Rus E, Santimbrean C, Mardare N, Covic A. The success story of peritoneal dialysis in Romania: analysis of differences in mortality by dialysis modality and influence of risk factors in a national cohort. Perit Dial Int. 2006;26(2):266–275. [PubMed] [Google Scholar]
- 32. ClinicalTrials.gov. IMPENDIA - PEN vs Dianeal only; improved metabolic control in diabetic CAPD and APD patients. U.S. National Institutes of Health.