Figure 1. The Chlamydia trachomatis infectious cycle and modulation of host cell functions.
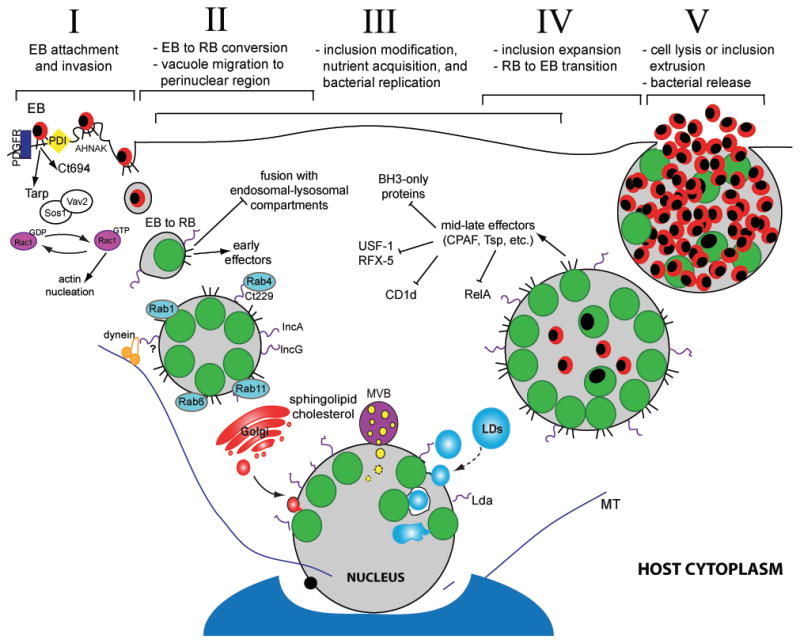
Chlamydia infection begins when an elementary body (EB) binds to the host cell surface (I). At this stage effector proteins injected into the host via the type III secretion system (T3SS) facilitate bacterial entry. Following endocytosis, EBs transition to reticulate bodies (RBs) (II). New effectors are secreted and the bacterial vacuole is modified by bacterial inclusion membrane proteins (Incs) to limit fusion with the host degradative compartments while promoting contact with other host organelles and factors, including Rab proteins. The inclusion interacts with the Golgi apparatus, multivesicular bodies (MVBs), and lipid droplets (LDs). LDs can be directly translocated into the inclusion lumen for nutrient delivery (III). During infection, host cell death and immune defenses are inhibited. Approximately midway through the infectious cycle, bacterial replication becomes asynchronous and RBs re-differentiate into EBs (IV). Late in the cycle, the inclusion is packed with EBs and fills almost the entire cell volume. Eventually, the inclusion and host cell rupture releasing infectious EBs into the extracellular space for reinfection (V).
