Abstract
γ-Secretase cleaves multiple substrates within the transmembrane domain that include the amyloid precursor protein as well as the Notch family of receptors. These substrates are associated with Alzheimer disease and cancer. Despite extensive investigation of this protease, little is known regarding the regulation of γ-secretase specificity. To discover selective inhibitors for drug development and for probing the mechanisms of γ-secretase specificity, we screened chemical libraries and consequently developed a di-coumarin family of inhibitors that preferentially inhibit γ-secretase-mediated production of Aβ42 over other cleavage activities. These coumarin dimer-based compounds interact with γ-secretase by binding to an allosteric site. By developing a multiple photo-affinity probe approach, we demonstrate that this allosteric binding causes a conformational change within the active site of γ-secretase at the S2 and S1 sub-sites that leads to selective inhibition of Aβ42. In conclusion, by using these di-coumarin compounds, we reveal a mechanism by which γ-secretase specificity is regulated and provide insights into the molecular basis by which familial presenilin mutations may affect the active site and specificity of γ-secretase. Furthermore, this class of selective inhibitors provides the basis for development of Alzheimer disease therapeutic agents.
Keywords: affinity labeling, Alzheimer disease, allosteric regulation, di-coumarin
γ-Secretase is a multi-protein membrane-bound complex that is currently at the front line of basic and translational research. It is composed of at least 4 proteins that include presenilin, nicastrin, Aph-1, and Pen-2 (1). Presenilin is believed to contain the active site of γ-secretase (2–4). It represents a novel class of protease that catalyzes peptide bond hydrolysis within the transmembrane hydrophobic environment and plays an essential role in a newly emerged signaling pathway known as regulated intramembrane proteolysis (5). γ-Secretase cleaves a variety of type I membrane proteins that include the amyloid precursor protein (APP) and the Notch family of proteins despite limited primary sequence homology across targeted substrates (6). Elucidation of the mechanisms that control the specificity of γ-secretase for these substrates has been hindered by technical difficulties associated with intramembrane enzymology. Determining the factors that contribute to γ-secretase specificity is critical to understanding the biology of this unique protease and targeting it for therapeutic purposes.
γ-Secretase has become an appealing drug target for Alzheimer disease (AD) and cancer because of its central role in the processing of APP and Notch (6). γ-Secretase cleaves APP to generate neurotoxic Aβ peptides, ranging from 37 to 46 aa in length (7). Among them, Aβ40 and Aβ42 have been extensively investigated for their association with AD (7). Additionally, disease-causing familial AD mutations within APP, presenilin-1 (PS-1), and presenilin-2 (PS-2) proteins result in an increase in the ratio of Aβ42 to Aβ40 (reviewed in ref. 7). Clearly, mutations in both enzyme and substrate can influence the specificity of γ-secretase and lead to pathological consequences. Non-selective inhibition of γ-secretase activity has been explored as an AD and cancer therapeutic approach, but the abrogation of all activities of γ-secretase results in toxicity in the gastrointestinal tract as a result of the blockage of Notch1 signaling (8). Therefore, the development of selective inhibitors is necessary to investigate γ-secretase specificity and provide candidates for drug development.
Recent studies have indicated that the ratio of Aβ42 to Aβ40, rather than the total amount of β-amyloid, correlates with the amount of characteristic AD plaques in mouse models (9, 10) as well as with the age of onset of familial AD (11). Furthermore, new evidence suggests that Aβ40 may even play a neuroprotective role against AD progression, whereas Aβ42 is more hydrophobic and more readily aggregates to form toxic oligomers and fibrils (10). Rationally, the discovery and development of selective γ-secretase inhibitors (GSIs) that specifically abrogate Aβ42 production over Aβ40 and Notch cleavage is a promising strategy for AD therapy.
Weggen et al. discovered that a subset of nonsteroidal antiinflammatory drugs, referred to as γ-secretase modulators (GSMs), were able to selectively decrease γ-secretase-mediated production of Aβ42 with a concomitant increase in Aβ38, and had no effect on Aβ40 or Notch1 cleavage (12). Conversely, other GSMs were determined to stimulate the production of Aβ42 while reducing Aβ38 cleavage. Subsequent studies have shown that these GSMs alter γ-secretase cleavage preference by binding directly to the APP substrate (13). Other compounds that target γ-secretase and preferentially inhibit Aβ40 and Aβ42 production over Notch1 processing have been reported (14, 15), although the precise action mechanism of these molecules has not been established. Therefore, it is critical to develop a better understanding of the molecular basis of γ-secretase specificity to facilitate the development of selective GSIs for the treatment of AD and other human disorders.
In the present study, we describe a class of allosteric GSI (AGSI) that contain a di-coumarin core and modulate γ-secretase specificity for Aβ42 production over Aβ38, Aβ40, and Notch cleavages. We have demonstrated that these inhibitors regulate γ-secretase activity by binding to an allosteric site within the γ-secretase complex. Furthermore, we have developed a multiple photo-affinity probe strategy using transition-state inhibitors that allows us to evaluate the architecture of the active site of γ-secretase. Using this method we demonstrate that the binding of di-coumarin compounds to γ-secretase causes a conformational change in the S1 and S2 sub-sites, which may explain the selective regulation of protease by these small molecules. This work offers evidence of a molecular mechanism by which γ-secretase specificity is modulated by small probes and could potentially explain how certain PS1 familial mutations influence AD. In conclusion, these inhibitors represent important tools that will help elucidate factors contributing to γ-secretase specificity and its relationship to AD.
Results
Di-Coumarin Compounds Are Selective GSIs in Vitro.
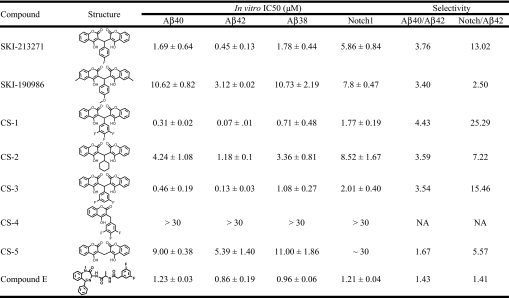
To discover selective GSIs, we screened large collections of small molecules (approximately 200,000 compounds) at the Sloan-Kettering Institute High Throughput Screening (HTS) core facility. Our HTS approach uncovered several novel classes of GSIs as well as currently established scaffolds. Among them, the presented class contains a symmetric di-coumarin core joined by a central benzene ring that displays specificity against Aβ42 production. The HTS screen revealed 5 inactive compounds in this structural class and 2 active hits: SKI-213271 and SKI-190986. In our multiple in vitro assays, both compounds selectively abrogated Aβ42 production over Aβ40 (Table 1) by approximately 3.5-fold. Additionally, we determined that both lead compounds did not promote Aβ38 production, which is distinct from the previously reported GSMs (12). Last, the coumarin-dimer compounds also exhibited decreased potency for inhibition of Notch-1 processing. Clearly, these compounds could represent a class of inhibitors that selectively target Aβ42 production. To develop more potent and selective inhibitors, we synthesized more than 40 analogs and have profiled a few in Table 1 with the respective IC50 values for each in vitro assay listed. The predominant trend for this family of compounds was increased potency against Aβ42 over Aβ40, Aβ38, or Notch. The most effective compound, CS-1, exhibited in vitro IC50 values of 0.07 μM, 0.31 μM, 0.71 μM, and 1.77 μM against Aβ42, Aβ40, Aβ38, and Notch, respectively. The inactivity of CS-4 suggests that the coumarin-dimer structure is necessary for inhibitory potency. Conversely, compound E, a potent pan-GSI, did not exhibit any significant selectivity for any of the cleavage activities assayed (Table 1). Preliminary structure-activity relationship analyses showed that the mono-, di- and tri-fluorobenzene rings incrementally increased the potency and selectivity of the compounds. Substitution of the fluorobenzene moiety with cyclohexane (CS-2) or hydrogen (CS-5) significantly reduced the potency and selectivity (Table 1). Furthermore, we tested the ability of CS-1 to retain its selectivity against γ-secretase from mouse brain membrane and found that it did maintain its preference for Aβ42 inhibition (IC50: Aβ40, 380 nM ± 35; Aβ42, 112 nM ± 40). Last, we also determined the inhibitory potency of CS-1 against cell membrane prepared from cells that stably express the PS1-M146L familial mutation (16). The IC50 values of CS-1 are 167 nM ± 21 and 206 nM ± 57 for Aβ40 and Aβ42, respectively.
Table 1.
In vitro characterization of coumarin-dimer allosteric GSIs against various γ-secretase cleavage products
The potency of 7 unique coumarin-based γ-secretase inhibitors were evaluated for efficacy against γ-secretase-mediated production of Aβ40, Aβ42, Aβ38, and Notch. Additionally, the pan-GSI compound E was also examined in these assays. The IC50 values were calculated from the dose–response curves using a non-linear regression analysis in Prism software. IC50 values are presented with SD (n = 3 for each data point). The 3 β-amyloid-detection in vitro assays were modified from our previously reported assay (21) using a biotinylated substrate that eliminated the requirement of anti-β-amyloid biotinylated antibody. Ruthenylated antibodies that detected the −40, −42, or −38 cleavage site were incorporated to detect proteolysis indicative of γ-secretase activity. In vitro Notch assay used a recombinant transmembrane portion of the Notch peptide and anti-Notch1 SM320 antibody in conjunction with ruthenylated anti-rabbit secondary antibodies. Electrochemiluminescence was quantified on an Analyzer (BioVeris). The selectivity ratio for Aβ42 inhibition over Aβ40 and Notch are indicated in the 2 far right columns.
Di-Coumarin Compounds Are Selective GSIs in Cells.
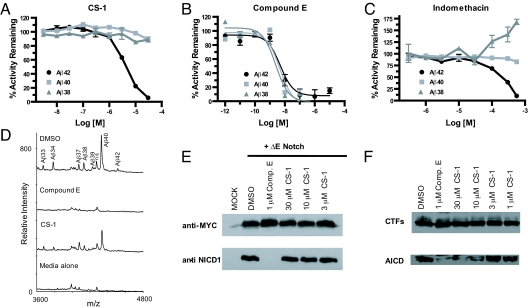
We next set out to determine if the selective inhibition of Aβ42 was maintained in a cell-based system for APP processing. First, we compared our lead compound CS-1 (Fig. 1A) with compound E (Fig. 1B) and the GSM compound indomethacin (Fig. 1C). N2a mouse neuroblastoma cells that stably express Swedish-mutated APP substrate were treated with the indicated compounds for 24 h at 37 °C. Following a 24-h incubation period, the media were collected from the cells and assayed for secreted Aβ42, Aβ40, and Aβ38. CS-1 inhibited Aβ42 production with an EC50 of approximately 3 μM in our cell-based assay, yet had virtually no effect on Aβ38 or Aβ40 production up to 30 μM (Fig. 1A). Furthermore, cytotoxicity studies using Alamar blue indicated CS-1 had little to no effect on cell viability up to 30 μM. In addition, we found that CS-3 exhibited an identical inhibitory profile with a slightly increased EC50 for Aβ42 inhibition (approximately 5 μM). Compound E inhibited the production of all 3 β-amyloid species with equal potency (Fig. 1B), whereas indomethacin significantly enhanced Aβ38 production, abrogated Aβ42, and had no effect on Aβ40 (Fig. 1C). The result for indomethacin mirrored those findings by Kukar et al. for which a different cell-based system was used (17), further validating our assay system for analysis of these Aβ species. We next confirmed these findings using immunoprecipitation-mass spectrometry (IP-MS), which revealed that CS-1 was able to inhibit Aβ42 while leaving Aβ38 and Aβ40 production largely intact (Fig. 1D). In a cell system, the coumarin-dimer based compounds retained their selectivity and exhibited an even greater specificity for inhibition of γ-secretase activity for Aβ42 production, which is a promising finding for drug development. This may reflect subtle variations between the cellular and in vitro conformations of γ-secretase. Nevertheless, the cell-based studies confirmed that CS-1 maintains a preference for inhibition of the γ-secretase mediated production of Aβ42 over Aβ40 or Aβ38, which is distinct from previously reported GSMs (17) and inhibitors (14, 15, 18).
Fig. 1.
Cellular evaluation of the coumarin-dimer CS-1 and its selective inhibition of Aβ42. Compounds were incubated with the APPsw-N2A mouse neuroblastoma cells for 24 h and media were analyzed by biotinylated 4G8 and ruthenylated antibodies specific for each respective cleavage product. (A) CS-1 preferentially abrogates Aβ42 production with no effect on Aβ40 or Aβ38. (B) The GSI compound E exhibits no inhibitory selectivity for inhibition of β-amyloid peptides. (C) The GSM indomethacin reduces Aβ42 production, potently increases Aβ38, and has little effect on Aβ40. (D) IP-MS analysis of CS-1 effect on secreted β-amyloid species. Aβ peptides were immunoprecipitated using 4G8 antibody and isolated with Protein G+/A agarose beads. Samples were analyzed by MALDI-MS. Samples shown are representative and each data point was performed in triplicate. (E) Cell-based Notch cleavage assay. HEK-293 cells were transfected with ΔE Notch construct and then compound E and CS-1 were evaluated for their ability to inhibit γ-secretase-mediated Notch intracellular domain production. Compound E inhibitor was able to prevent production of NICD, but CS-1 did not affect this cleavage. Western blot is representative and was performed in triplicate. (F) Effect of CS-1 on AICD production. N2A APPsw cell membrane was prepared and incubated with the indicated concentrations of CS-1 at 37 °C for 2 h. The generated AICD and APP-CTFs were detected by Western blotting using APPc antibody. Western blot is representative and was performed in triplicate.
We next determined the ability of CS-1 to suppress cellular γ-secretase activity for Notch1 cleavage. The ΔE Notch construct encodes a truncated Notch1 protein that lacks the majority of the extracellular domain and no longer requires ligand binding or S2 cleavage (19). The fragment expressed by the ΔE Notch construct is a membrane-tethered portion of the Notch-1 receptor that is a direct substrate of γ-secretase. ΔE Notch was transiently expressed in HEK-293 cells for 24 h in the presence of DMSO or GSI. The expression of ΔE Notch protein was confirmed by anti-Myc antibody. We found that compound E effectively blocked all production of the Notch intracellular domain (NICD) as detected by the anti-NICD1 SM320 antibody. However, CS-1 at concentrations up to 30 μM, which was able to abrogate virtually all of Aβ42 production, had no effect on NICD generation (Fig. 1E). In addition, we examined the potency of CS-1 on APP intracellular domain (AICD) production and determined that it is less potent for this cleavage with an IC50 of more than 10 μM (Fig. 1F). This result further highlights the selectivity of this class of coumarin-dimer compound for Aβ42 inhibition.
Di-Coumarin Inhibitors Are Non-Competitive Inhibitors.
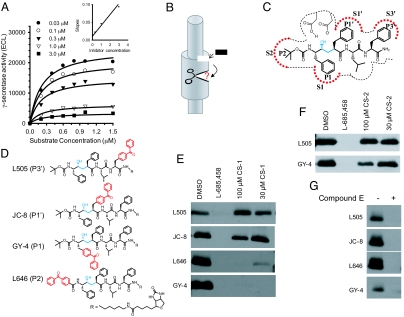
Following the realization that CS-1 and its analogs were exhibiting an in vitro and cell-based selectivity for Aβ42 over other γ-secretase cleavage activities, we examined their mechanism of action. Inhibition kinetic analysis of CS-1 showed that it affects Vmax but not Km, indicating non-competitive inhibition against the APP-transmembrane domain substrate (Fig. 2A), whereas L-685,458 (L458), a transition state inhibitor (20), behaves as a competitive inhibitor against the same substrate. The findings regarding L458 were consistent with our previous report (21). Additionally, the re-plotting of slope against inhibitor concentration shows a linear relationship (R2 = 0.98; Fig. 2A Inset), suggesting a purely non-competitive inhibition and a single inhibitor binding site. It is noteworthy to point out that L458 acts as a non-competitive inhibitor when the C100 substrate is used as a result of a putative docking site interaction (22). The non-competitive behavior of this class of inhibitors against APP-transmembrane domain substrate suggests that the coumarin dimer compounds are binding to γ-secretase at an allosteric site and thereby preventing enzyme activity.
Fig. 2.
Kinetic analysis of AGSIs and evaluation of their effect on the γ-secretase active site architecture. (A) Kinetic analysis of CS-1 was performed using our modified version of a previously reported in vitro γ-secretase activity assay (21). The inhibition kinetics were analyzed by using a non-linear curve fit with the Michaelis-Menten equation. Inset (Upper Right): We re-plotted slopes against the inhibitor concentrations after performing double reciprocal conversion. (B) Schematic representation of the allosteric binding of the di-coumarin compounds to γ-secretase. This binding ultimately causes an alteration at the active site of γ-secretase. Black rectangle represents the coumarin-dimer compound. (C) The binding of L458 to the active site of γ-secretase and its interaction at various sub-pockets within the enzyme. (D) Chemical structure of the 4 photo-affinity probes used in the characterization of CS-1 effect on active site architecture. Hydroxyethylamine and benzophenone moieties are marked by blue and red, respectively. (E) Evaluation of CS-1 effect on the photo-labeling of 4 probes. CS-1 has little to no effect on the ability of JC-8 and L505 to label the active site at the S1′ and S3′ sites, respectively. CS-1 blocks photo-incorporation of the benzophenone group of the L646 and GY-4 compounds that label the S2 and S1 sub-sites, respectively. (F) Evaluation of CS-2 effect on the active site photo-labeling by L505 and GY4. (G) Effect of compound E on active site photo-labeling. Compound E at 2 μM completely suppressed photo-labeling of all 4 probes. Blotting was performed for PS1-NTF. The photo-labeling blots are representative and were performed in triplicate.
Di-Coumarin Inhibitors Alter the Sub-Sites of the γ-Secretase Active Site.
We hypothesized that the allosteric binding of the di-coumarin compounds alters the conformation of the active site of γ-secretase and thereby preferentially affects the Aβ42 site cleavage (Fig. 2B). This raised the technical issue of how to probe the contours of the enzymatic active site. Although the structure of γ-secretase has been determined by cryo-electron microscopy (23), the resolution attained is not sufficient to investigate subtle changes within the active site. Consequently, we developed a series of active site-directed inhibitors that incorporate a photoreactive benzophenone entity into varied positions. Using these photoreactive probes, we assessed the effect of the di-coumarin inhibitor binding on the active site of γ-secretase. As the efficiency of photo-insertion depends on the orientation of the probe and the proximity of residues within the active site, conformational change of the active site can alter the orientation of the probe and contact residues and lead to altered cross-linking efficiencies. Therefore, multiple photo-activatable, active site-directed GSIs will provide a practical approach to evaluate the changes within the active site following allosteric di-coumarin binding.
L458 contains a hydroxyethylamine transition-state isostere that mimics the tetrahedral intermediate of aspartyl proteases and this moiety hydrogen bonds with the catalytic aspartate residues of γ-secretase (20). According to the nomenclature of Schechter and Berger (24), L458 contains the P2, P1, P1′, P2′, and P3′ residues that putatively bind to the S2, S1, S1′, S2′, and S3′ sub-sites, respectively, within the active site of γ-secretase (Fig. 2C). We have developed a series of biotinylated, photo-activatable inhibitors based on the core structure of L458 that allow us to probe the sub-pockets of the γ-secretase active site (3, 25, 26). These inhibitors all have an individual benzophenone group incorporated into L458 at the P2, P1, P1′, or P3′ position and are referred to as L646, GY4, JC8, and L505 (Fig. 2D). Each of these inhibitors interacts and labels the S2, S1, S1′, and S3′ sub-sites, respectively, within the γ-secretase complex (Fig. 2 C and D).
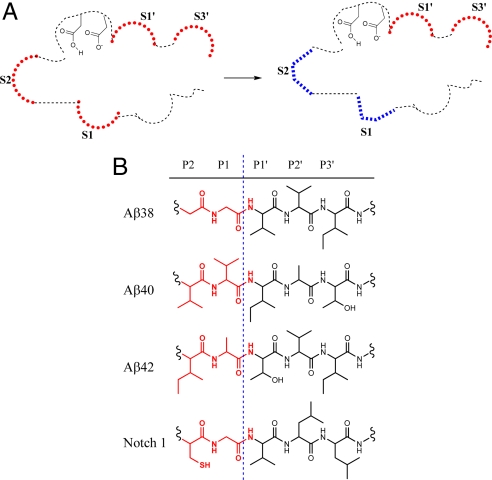
HeLa membrane was incubated with CHAPSO detergent and photo-affinity probe in the presence or absence of excess L458 or CS-1. Labeled presenilin was isolated using streptavidin beads, separated by SDS/PAGE, and subsequently Western blotted using anti-PS1-NTF antibodies. Again, presenilin is believed to contain the active site of γ-secretase, so we examined PS1 photo-labeling. We determined that the compounds each labeled PS1-NTF, which migrated at approximately 34 kDa (Fig. 2E). First, as expected, excess L458 at 2 μM completely blocked photo-insertion of each probe. This demonstrated that the active site photo-labeling was specific (Fig. 2E). Second, CS-1 up to 100 μM did not block the L505 labeling of PS1-NTF and only slightly inhibited JC-8. This indicated that CS1 binding has no significant effect on the S1′ and S3′ sub-sites and supports the notion that CS-1 and L458 do not bind at the same site within γ-secretase (Fig. 2E Upper). Third, CS-1 virtually abolished all of the labeling of PS1-NTF by L646 and GY-4 (Fig. 2E Lower), which confirmed that this class of inhibitors directly interacts with γ-secretase and that CS-1 binding alters the S2 and S1 sub-pockets within the active site. Moreover, CS-2, which is 17-fold less potent than CS-1 for Aβ42 inhibition (Table 1), did not alter L505 photo-labeling of the S3′ sub-site and only partially blocked GY-4 labeling at 100 μM (Fig. 2F). Clearly, inhibition of the photo-insertion of GY-4 is related to the potency of these allosteric GSI (AGSI) compounds. Last, compound E at 2 μM non-selectively blocked photo-insertion of all 4 probes (Fig. 2G). Taken together, these results indicate that the binding of CS-1 to an allosteric site in γ-secretase alters the active site architecture, mainly affecting the S2 and S1 (i.e., non-prime side) sub-sites (Fig. 3A). It is possible that CS-1-induced conformational changes within the active site of γ-secretase alter the enzymatic interaction with the P2 and P1 residues of Aβ42 (Ile-Ala), yet minimally affect the P2 and P1 side chains of Aβ38, Aβ40, or Notch-1 (Gly-Gly, Val-Val, and Cys-Gly, respectively; Fig. 3B). Regardless, it is clear that these di-coumarin AGSIs selectively abolish Aβ42 cleavage over Aβ38, Aβ40, and Notch1, and this selectivity is likely a result of alteration within the S2 and S1 pockets of the enzymatic active site.
Fig. 3.
Di-coumarin binding alters the active site of γ-secretase and preferentially alters Aβ42 cleavage. (A) Schematic representation of the AGSI effect on the γ-secretase active site-binding pockets. Binding of CS-1 alters the S1 and S2 sub-sites within the active site of γ-secretase that were probed by GY-4 and L646, respectively, and ultimately leads to a selective inhibition of Aβ42. Active site conformational change is depicted by a change in shape and color at the S1 and S2 sub-sites. (B) The P2-P3′ residues of Aβ38, Aβ40, Aβ42, and Notch. Alteration of the S2 and S1 sub-sites may influence Aβ42 production more significantly than other cleavages.
Discussion
γ-Secretase cleaves numerous substrates that are involved in diverse biological processes. The multiple substrates of γ-secretase appear to possess little primary sequence homology, and consequently, the factors governing cleavage specificity remain unknown. The localization or compartmentalization of γ-secretase substrates has been proposed as one mechanism to control its activity (27, 28). In addition to processing multiple proteins, γ-secretase initiates proteolysis of APP at multiple sites. Among the products that result, Aβ42 is more hydrophobic and therefore more prone to aggregate and form the characteristic neurotoxic oligomers and fibrils associated with AD compared with other β-amyloid species (29). Therefore, factors that promote the generation of Aβ42 are believed to accelerate the pathological cascade leading to AD. Mutations in APP, PS-1, and PS-2 are linked to familial forms of early-onset AD (7). The majority of mutations within each of these genes cause an increase in the ratio of Aβ42 to Aβ40 in biochemical, cellular, and animal models. Recent studies suggest that alteration of γ-secretase complex dynamics and/or formation of γ-secretase complexes with mutated components can affect the enzymatic cleavage specificity (30, 31). Despite these advances in our understanding, little is known regarding the molecular mechanisms that control the specificity of γ-secretase-mediated cleavage at the Aβ40, Aβ42, or Notch1 cleavage locations. Our work has provided the first evidence that changes in the active site architecture can modulate γ-secretase specificity and provides a rationale for the design of selective GSIs targeting the S2 and S1 sub-sites. Additionally, we present a family of small molecule inhibitors that can be used to probe the biology of γ-secretase and may serve as the basis for AD drug development.
First, developing GSIs that preferentially abrogate Aβ42 production over other Aβ species or substrates has been an appealing strategy for AD therapeutics. Establishment of these selective inhibitors could potentially reduce the Notch-related toxicity witnessed with current GSIs and maintain Aβ40 production, which is thought to be neuroprotective against AD (10). In this study, we have identified a coumarin-dimer class of AGSIs that preferentially inhibit γ-secretase-mediated Aβ42 generation over Aβ40, Aβ38, or Notch in vitro as well as in cell-based systems. These AGSIs directly target γ-secretase by binding to an allosteric site within the enzyme, rather than targeting the APP substrate. Furthermore, these coumarin-dimer compounds similarly affect γ-secretase activity for Aβ40 and Aβ38 production and lack the interconnected effect witnessed with the GSMs in which decreased Aβ42 resulted in increased Aβ38 generation, and vice versa (17). Therefore, these AGSIs represent a class of inhibitors that are distinct from the GSMs (12, 17) as well as previously reported GSIs (14, 15, 18). It is noteworthy to point out that coumarin-dimer based compounds have been reported to be active against HIV integrase (32) and human NAD(P)H:quinine oxidoreductase-1 (33), as well as exhibit anticoagulant activity (34). However, the coumarin-dimer compounds that Nolan et al. reported (33) that are most potent against NAD(P)H:quinine oxidoreductase-1 lack the central benzene ring (CS-5) and therefore exhibit a much weaker inhibition of γ-secretase (Table 1). Clearly, these compounds possess a distinct structure and activity relationship against NAD(P)H:quinine oxidoreductase-1 compared with γ-secretase. Therapeutic application of these AGSI compounds needs to be further investigated. Additionally, we have demonstrated that AGSIs bind to an allosteric site within the γ-secretase complex, thereby influencing the interaction of γ-secretase with our active site-directed inhibitors. The presented data reveals that AGSI binding is capable of altering the conformation of the catalytic core of γ-secretase within the S2 and S1 sub-sites. These changes likely are the cause for differential inhibition of Aβ42 over Aβ38, Aβ40, and Notch cleavage by the di-coumarin compounds. Therefore, it is conceivable that other factors influencing γ-secretase cleavage specificity for Aβ42 could similarly affect the S2 and S1 pockets. PS-1 familial AD mutations significantly affect Aβ42 production and represent one potential pathological example in which mutational alteration of the S2 and S1 sub-sites results in altered enzymatic specificity.
Finally, we have developed a rational method to monitor subtle changes in the conformation of the γ-secretase active site using photo-activatable, active site-directed probes. γ-Secretase is a large multi-protein complex composed of at least 4 proteins possessing 19 putative transmembrane domains. The complexity of γ-secretase has made acquisition of its crystal structure a formidable challenge, and it has not yet been successfully obtained. Our method therefore offers a practical chemical approach for elucidating the action mechanism of inhibitors against the γ-secretase complex and other enzymes in which sufficient resolution of structures are not available or obtainable. These photoreactive compounds are valuable tools for examining the active site of endogenous γ-secretase and can be used to analyze factors that influence its conformation or to investigate differences across varied tissues or cell lines.
In summary, the discovery of these selective AGSIs and development of our multiple photo-affinity small molecule approach has helped to elucidate a mechanism of γ-secretase specificity and shed light on how γ-secretase specificity is modulated. Furthermore, the family of di-coumarin compounds represents a class of drug candidates for AD therapeutic drug development and will be useful probes for unraveling the intricacies of this enigmatic protease under physiological and pathological conditions.
Materials and Methods
Reagents, GSIs, and Photo-Affinity Probes.
Coumarin-based GSIs were synthesized in our laboratory and will be described in detail elsewhere, whereas compound E was synthesized as previously described (35). The syntheses of L458, L646, L505 (3), GY-4 (25), and JC-8 (26) were all previously described elsewhere. The polyclonal anti-NICD-1 SM320 antibody that was produced using a peptide antigen was purified using peptide antigen immobilized resin.
In Vitro and Cell-Based γ-Secretase Assays.
Cell membranes and solubilized γ-secretase were prepared as described previously (36). The in vitro and cell γ-secretase assays detecting Aβ38, Aβ40, or Aβ42 cleavage were performed similarly as previously described (21, 36). Cleaved product was detected using ruthenylated antibodies that recognize specific APP cleavage sites (Aβ1–38*, G2–10*, or G2–11* antibody for Aβ38, Aβ40, or Aβ42, respectively). The Km and Vmax in the presence and absence of GSIs were analyzed by non-linear curve fit using SigmaPlot 8.0 software with the Michaelis-Menten equation (ν = Vm [S]/(Km + [S]; ν, initial rate; Vm, maximum velocity; Km, Michaelis-Menten constant; S, substrate).
The in vitro γ-secretase assay detecting Notch cleavage was similar to the assays described earlier, with a few notable differences. First, the substrate used was a directly biotinylated Notch transmembrane domain peptide (Notch1-TM, acetyl-YVAAAAFVLLFFVGCGVLLSRKRRRQHGK-biotin). This Notch substrate was incubated with 40 ng/μL solubilized γ-secretase, 0.25% CHAPSO, and 1% DMSO or GSI in the presence of 1× Pipes, pH 7.0, buffer for 2.5 h at 37 °C. Cleaved product was detected using the affinity polyclonal anti-NICD-1 antibody (SM320), which recognizes the cleaved product and not the substrate, as well as a ruthenylated secondary anti-rabbit antibody. The sample was then similarly incubated with magnetic streptavidin beads and quantified by measuring electrochemiluminescence.
IP-MS Analysis of β-Amyloid Peptides from Cell Media.
Aβ peptide profiles were analyzed by IP/MS (37). Aliquots of 1.0 mL conditioned media (DME-HG, Opti-Mem, 10% FBS, Pen/Strep, G418) from N2A mouse neuroblastoma cells overexpressing APP Swedish mutation were immunoprecipitated by monoclonal antibody 4G8 and Protein G+/A agarose beads in the presence of internal standard, Aβ12–28 (10 nM). Aβ peptides were extracted from the beads with α-cyano-4-hydroxycinnamic acid matrix (using formic acid/water/isopropanol 1:4:4 vol/vol/v as solvent) and spotted on a MALDI target plate prepared by the thin-layer method. The molecular masses of immunoprecipitated Aβ species were measured using a Voyager-DE STR MALDI-TOF MS (Applied Biosystems). Each spectrum was collected using 750 laser shots. Mass spectra were calibrated using bovine insulin as internal mass calibrant. Peaks corresponding to Aβ peptides were identified using the measured molecular masses searching against human Aβ peptide.
Cell-Based Notch Cleavage Assay.
ΔE Notch or empty pcDNA3.1(-) construct was transfected into HEK-293 cells in a 6-well format using Lipofectamine reagent, following manufacturer's instructions. Transfection mixture was incubated with cells for 5 h at 37 °C. Following incubation, media were removed and fresh media were added back, containing 1% DMSO or GSI. This was incubated for 24 h at 37 °C, after which the cells were washed once in PBS solution and lysed in 1× radioimmunoprecipitation assay buffer [50 mM Tris, pH 8.0, 150 mM NaCl, 0.1% (wt/vol) SDS, 1% (vol/vol) Nonidet P-40, and 0.5% (wt/vol) deoxycholic acid] containing protease inhibitors. Samples were then centrifuged at 13,000 × g at 4 °C and the supernatant was collected and analyzed by Western analysis using anti-Myc antibody at a 1:1,000 dilution or anti-NICD-1 SM320 at a 1:500 dilution.
AICD Generation Assay and Photo-Labeling γ-Secretase Active Site.
The generation of AICD by γ-secretase was performed as previously described (38) using N2A mouse neuroblastoma cells stably overexpressing the APP Swedish mutation (N2A APPsw). Photo-labeling experiments are performed as previously described (3).
Acknowledgments.
We thank M. Lai for providing the PS1-NTF antibody and R. Kopan for providing the ΔE Notch-1 construct. We are grateful to S. Gross and D. Scheinberg for helpful discussion and analysis of the research, and G. Dolios for assistance performing IP-MS analysis of samples. We thank L. Placanica for critical analysis of the manuscript and G. Sukenick and S. Rusli (Nuclear Magnetic Resonance Core Facility, Sloan-Kettering Institute) for mass spectral analyses. The authors are also grateful to D. Shum and other members of the HTS Core Facility for their help during the course of this study. This work is supported by the Mr. W. H. Goodwin and Mrs. A. Goodwin and the Commonwealth Foundation for Cancer Research (to Y.M.L. and H.D.), The William Randolph Hearst Foundation (to Y.M.L. and H.D.), The Lillian S. Wells Foundation (to H.D.), and the Experimental Therapeutics Center (to Y.M.L. and H.D.) of Memorial Sloan-Kettering Cancer Center; National Institutes of Health (NIH) Grants R01-AG026660 (to Y.M.L.) and R01-AG20670 (to H.Z.); NIH/National Center for Research Resources Grant S10 RR022415 (to R.W.); NIH National Research Service Award pre-doctoral fellowship 5F31NS053218 (to C.C.S.); and the Alzheimer's Association (to Y.M.L. and R.W.).
Footnotes
The authors declare no conflict of interest.
References
- 1.De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe MS, et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 3.Li YM, et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 4.Esler WP, et al. Transition-state analogue inhibitors of gamma-secretase bind directly to presenilin-1. Nat Cell Biol. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- 5.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 6.Kopan R, Goate A. A common enzyme connects notch signaling and Alzheimer's disease. Genes Dev. 2000;14:2799–2806. doi: 10.1101/gad.836900. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Alzheimer's disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 8.van Es JH, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 9.Deng Y, et al. Deletion of presenilin 1 hydrophilic loop sequence leads to impaired gamma-secretase activity and exacerbated amyloid pathology. J Neurosci. 2006;26:3845–3854. doi: 10.1523/JNEUROSCI.5384-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, et al. Abeta40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27:627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar-Singh S, et al. Mean age-of-onset of familial alzheimer disease caused by presenilin mutations correlates with both increased Abeta42 and decreased Abeta40. Hum Mutat. 2006;27:686–695. doi: 10.1002/humu.20336. [DOI] [PubMed] [Google Scholar]
- 12.Weggen S, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 13.Kukar TL, et al. Substrate-targeting gamma-secretase modulators. Nature. 2008;453:925–929. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer SC, et al. Discovery of begacestat, a Notch-1-sparing gamma-secretase inhibitor for the treatment of Alzheimer's disease. J Med Chem. 2008;51:7348–7351. doi: 10.1021/jm801252w. [DOI] [PubMed] [Google Scholar]
- 15.Netzer WJ, et al. Gleevec inhibits beta-amyloid production but not Notch cleavage. Proc Natl Acad Sci USA. 2003;100:12444–12449. doi: 10.1073/pnas.1534745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borchelt DR, et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 17.Kukar T, et al. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat Med. 2005;11:545–550. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- 18.Harrison T, Churcher I, Beher D. gamma-Secretase as a target for drug intervention in Alzheimer's disease. Curr Opin Drug Discov Devel. 2004;7:709–719. [PubMed] [Google Scholar]
- 19.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 20.Shearman MS, et al. L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid beta-protein precursor gamma-secretase activity. Biochemistry. 2000;39:8698–8704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- 21.Yin YI, et al. {gamma}-secretase substrate concentration modulates the Abeta42/Abeta40 ratio: Implications for Alzheimer disease. J Biol Chem. 2007;282:23639–23644. doi: 10.1074/jbc.M704601200. [DOI] [PubMed] [Google Scholar]
- 22.Tian G, et al. Linear non-competitive inhibition of solubilized human gamma-secretase by pepstatin A methylester, L685458, sulfonamides, and benzodiazepines. J Biol Chem. 2002;277:31499–31505. doi: 10.1074/jbc.M112328200. [DOI] [PubMed] [Google Scholar]
- 23.Osenkowski P, et al. Cryoelectron microscopy structure of purified gamma-secretase at 12 A resolution. J Mol Biol. 2009;385:642–652. doi: 10.1016/j.jmb.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 25.Yang G, et al. Stereo-controlled synthesis of novel photoreactive gamma-secretase inhibitors. Bioorg Med Chem Lett. 2009;19:922–925. doi: 10.1016/j.bmcl.2008.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun J, Yin YI, Yang G, Tarassishin L, Li YM. Stereoselective synthesis of photoreactive peptidomimetic gamma-secretase inhibitors. J Org Chem. 2004;69:7344–7347. doi: 10.1021/jo0486948. [DOI] [PubMed] [Google Scholar]
- 27.Tarassishin L, Yin YI, Bassit B, Li YM. Processing of Notch and amyloid precursor protein by gamma-secretase is spatially distinct. Proc Natl Acad Sci USA. 2004;101:17050–17055. doi: 10.1073/pnas.0408007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vetrivel KS, et al. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J Biol Chem. 2005;280:25892–25900. doi: 10.1074/jbc.M503570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarrett JT, Berger EP, Lansbury PT., Jr The C-terminus of the beta protein is critical in amyloidogenesis. Ann N Y Acad Sci. 1993;695:144–148. doi: 10.1111/j.1749-6632.1993.tb23043.x. [DOI] [PubMed] [Google Scholar]
- 30.Placanica L, et al. Pen2 and presenilin-1 modulate the dynamic equilibrium of presenilin-1 and presenilin-2 {gamma}-secretase complexes. J Biol Chem. 2009;284:2967–2977. doi: 10.1074/jbc.M807269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentahir M, et al. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem. 2006;96:732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, et al. Coumarin-based inhibitors of HIV integrase. J Med Chem. 1997;40:242–249. doi: 10.1021/jm960450v. [DOI] [PubMed] [Google Scholar]
- 33.Nolan KA, et al. Coumarin-based inhibitors of human NAD(P)H:quinone oxidoreductase-1. Identification, structure-activity, off-target effects and in vitro human pancreatic cancer toxicity. J Med Chem. 2007;50:6316–6325. doi: 10.1021/jm070472p. [DOI] [PubMed] [Google Scholar]
- 34.Zhou HY, Hong JL, Shu P, Ni YJ, Qin MJ. A new dicoumarin and anticoagulant activity from Viola yedoensis Makino. Fitoterapia. 2009;80:283–285. doi: 10.1016/j.fitote.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Lammich S, et al. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide. J Biol Chem. 2002;277:44754–44759. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- 36.Li YM, et al. Presenilin 1 is linked with gamma-secretase activity in the detergent solubilized state. Proc Natl Acad Sci USA. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R, Sweeney D, Gandy SE, Sisodia SS. The profile of soluble amyloid beta protein in cultured cell media. Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J Biol Chem. 1996;271:31894–31902. doi: 10.1074/jbc.271.50.31894. [DOI] [PubMed] [Google Scholar]
- 38.Hecimovic S, et al. Mutations in APP have independent effects on Abeta and CTFgamma generation. Neurobiol Dis. 2004;17:205–218. doi: 10.1016/j.nbd.2004.04.018. [DOI] [PubMed] [Google Scholar]