Abstract
Background
Treatment for tuberculosis (TB) is common among individuals receiving stavudine-containing highly active antiretroviral therapy (HAART), but the effect of TB treatment on stavudine toxicity has received little attention. We estimated the effect of TB treatment on risk of stavudine substitution among individuals receiving first-line HAART.
Methods
We evaluated a cohort of 7,066 patients who initiated HAART between April 2004 and March 2007 in Johannesburg, South Africa. Three exposure categories were considered: ongoing TB treatment at HAART initiation; concurrent initiation of TB treatment and HAART; incident TB treatment after HAART initiation. The outcome was single-drug stavudine substitution. Adjusted hazard ratios (aHRs) were estimated using marginal structural models to control for confounding, loss to follow-up, and competing risks.
Results
Individuals with ongoing and concurrent TB treatment were at increased risk of stavudine substitution, irrespective of stavudine dose. For ongoing TB treatment, aHR was 3.18 (95% confidence interval [CI] 1.82-5.56) in the first two months of HAART, 2.51 (95% CI 1.77-3.54) in months 3-6, and 1.19 (95% CI 0.94-1.52) thereafter. For concurrent TB treatment, aHR was 6.60 (95% CI 3.03-14.37) in the first two months,1.88 (95% CI 0.87-4.09) in months 3-6, and 1.07 (95% CI 0.65-1.76) thereafter. There was no effect of incident TB on stavudine substitution risk.
Conclusions
Risk of stavudine substitution was increased among patients receiving TB treatment, especially soon after HAART initiation. In settings where alternative antiretroviral drugs are available, initiation of stavudine in patients receiving TB treatment may need to be reconsidered.
Keywords: Tuberculosis treatment, HIV, stavudine, highly active antiretroviral therapy (HAART), drug interactions
BACKGROUND
The rapid rollout of highly active antiretroviral therapy (HAART) to individuals living with human immunodeficiency virus (HIV) has expanded rapidly in the last five years, particularly in sub-Saharan Africa where access to HAART expanded from 100,000 in 2003 to 1.3 million people by the end of 2006 [1]. An important challenge faced by HIV/AIDS care programs is the management of adverse drug reactions in the face of limited human and diagnostic resources and limited choice of antiretroviral drugs [2-5]. In particular, the inclusion of the nucleoside reverse transcriptase inhibitor stavudine as a component of standard first-line HAART throughout sub-Saharan Africa remains controversial. While inexpensive, stavudine causes significant toxicity and has been implicated as a chief cause of symptomatic hyperlactatemia, lactic acidosis, lipid disorders, lipodystrophy, and peripheral neuropathy in various populations of HIV-positive individuals [3, 6-10]. In one study from South Africa, over 20% of patients required substitution of stavudine because of toxicity by 36 months, most often for reasons of lipodystrophy or peripheral neuropathy [3].
These toxicities may have significant implications for the success of HAART programs as they cause substantial morbidity [3, 10, 11], can impact adherence to HAART [2, 3], and may lead to treatment interruptions and virological failure [12]. While the risk factors for hyperlactatemia, the most clinically severe stavudine toxicity, have been well-characterized, the risk factors for all-cause stavudine toxicity resulting in substitution remain largely unexamined [3, 13, 14]. The impact of concomitant HAART and tuberculosis (TB) treatment on stavudine substitution remains almost entirely unexplored in sub-Saharan Africa where both TB and HIV are highly prevalent [15]. This is particularly concerning because isoniazid, a key component of standard first-line TB treatment [10, 16], has been associated with peripheral neuropathy, one of the most commonly reported stavudine toxicities [3, 10, 14, 17, 18]. We examined the effect of TB treatment on the incidence of all-cause stavudine substitution in a large public HIV/AIDS care clinic in Johannesburg, South Africa.
METHODS
Study site, clinic procedures, data collection
This study was conducted in the Themba Lethu Clinical Cohort, a cohort of adults initiating HAART at one of the largest public clinics providing HAART in South Africa. After initiating HAART in accordance with the South African National Comprehensive Care, Management and Treatment Guidelines for HIV and AIDS [16], patients are scheduled for clinical visits at 4 months and every 6 months thereafter, at which demographic, medical, and laboratory data are collected. All data were entered into a TherapyEdge-HIV™ (TherapyEdge Inc., USA) database and analyzed in SAS v9.1 (SAS Institute, Cary, NC, USA).
Patients who required TB treatment received a standardized course of directly observed therapy at primary health care clinics (outside of Themba Lethu Clinic) according to national treatment guidelines. Date of TB treatment initiation was obtained using TB patient clinic card and patient self-report to ensure accuracy. The standard first-line of TB treatment in South Africa is two months of isoniazid, rifampicin, pyramizade, and ethambutol followed by four months of isoniazid and rifampicin [16]. In South Africa, vitamin B6 (pyridoxine) is commonly prescribed at time of initiation of TB treatment. In the Themba Lethu Clinic, amitriptyline is commonly prescribed for individuals experiencing peripheral neuropathy [19].
Study eligibility and definitions
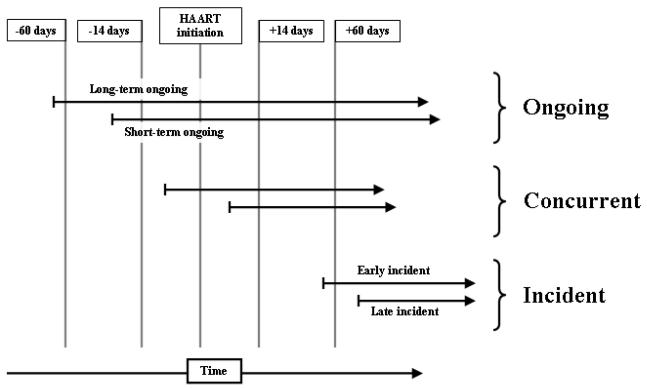
Patients were eligible for analysis if they initiated a stavudine-containing HAART regimen at Themba Lethu Clinic between 1 April 2004 to 31 March 2007. The exposure of interest was TB treatment, defined as patient treatment with isoniazid-containing therapy for pulmonary or extrapulmonary TB. We hypothesized that the effect of TB treatment on risk of stavudine substitution might be affected by the relative timing of HAART initiation and TB treatment; to this end we analyzed three exposure categories. Ongoing TB treatment was defined as TB treatment started at least 15 days before initiation of HAART, the minimum time advised by the National Guidelines and the WHO to wait between initiation of TB treatment and HAART [16, 20]. Concurrent TB treatment was defined as initiation of both TB treatment and HAART within 14 days of each other. Incident TB treatment was defined as initiation of TB treatment at least 15 days after initiation of HAART, a cutoff chosen to mirror the ongoing TB treatment category. A schematic diagram for these exposure categories is shown in Figure 1. For all exposures, the comparison group comprised individuals not receiving TB treatment. The outcome of all-cause stavudine substitution was defined as the event of substitution of a single drug (most often zidovudine) for stavudine while the rest of the regimen remained unchanged. Patients were followed up until they had the event of interest or until censoring at time of second episode of TB on HAART, death, loss to follow-up, multi-drug substitution, or end of follow-up (31 March 2007).
Figure 1.
Schematic diagram of TB treatment exposure categories in relation to time of HAART initiation (ongoing, concurrent, incident).
Statistical analyses
We performed an intent-to-treat analysis, controlling for confounding and censoring using inverse probability weighted marginal structural Cox proportional hazards models [21-23]. These methods are similar to Cox proportional hazards models, accounting for confounding by both baseline and time-varying factors where appropriate; in addition, these models can adjust analytically for losses to follow-up [24] and competing risks [25]. We controlled for confounding by gender, ethnicity, employment status, age, history of antiretroviral therapy, previous history of TB treatment, pregnancy, peripheral neuropathy at HAART initiation, hemoglobin (adjusted for sex, pregnancy, and altitude), body mass index (BMI), CD4 cell count, WHO stage, calendar date, whether treatment was initiated after October 2006 (when all consultation fees were eliminated), and stavudine dose (milligrams per kilogram).
In these analyses, patients became exposed at the earliest time they were receiving both HAART and TB treatment. However, we expected the effect of TB treatment to change with total time on both TB treatment and HAART. Time-interaction terms were included in all analyses to allow models to estimate these different effects by months of co-treatment: 0-2 months, 3-6 months, and 7+ months. Last, we performed several sensitivity analyses (described below). Further details of the statistical analysis are given in the Appendix.
Ethics approval
This study was approved by the human subjects review boards of both the University of the Witwatersrand and the University of North Carolina at Chapel Hill.
RESULTS
We analyzed 7,066 individuals who initiated stavudine-containing HAART, among whom there were a total of 1,845 cases of active TB. Of these cases, 1,272 were cases of ongoing TB treatment (716 for more than two months and 556 for less), 224 were cases of concurrent initiation of treatment, and 349 were cases of incident TB treatment (132 early, between 15 and 60 days of HAART initiation; and 217 more than 60 days after HAART initiation). Patients with either ongoing or concurrent TB treatment were less likely at time of HAART initiation to have a history of TB, and were more likely to be male and have advanced disease (lower CD4 counts, low BMI, low hemoglobin, more WHO stage IV disease) compared to those who not receiving TB treatment, and were more likely to receive a reduced (30mg) dose of stavudine (Table 1). Patients who later developed TB likewise had lower baseline BMI, CD4 count, and hemoglobin than those not receiving TB treatment at baseline, and were similarly more likely to receive a reduced dose of stavudine.
Table 1.
Baseline characteristics of 7066 individuals at time of HAART initiation in Johannesburg, South Africa, by timing of TB treatment status as it relates to HAART initiation. Figures expressed as n(%) except where noted.
| No TB treatment (n=5226) |
Ongoing TB treatment (n = 1272) |
Concurrent TB treatment (n = 224) |
Incident TB treatment (n = 344) |
|
|---|---|---|---|---|
| Pulmonary TB | n/a | 964 (75.8) | 164 (73.2) | 275 (79.9) |
| History of TB | 792 (15.2) | 64 (5.0) | 13 (5.8) | 62 (18.0) * |
| Stavudine dose | ||||
| 40 mg | 2879 (55.1) | 515 (40.5) | 89 (39.7) | 150 (43.6) |
| 30 mg | 2184 (41.8) | 743 (58.4) | 130 (58.0) | 185 (53.8) |
| Missing | 163 (3.1) | 14 (1.1) | 5 (2.2) | 9 (2.6) |
| Female gender | 3634 (69.5) | 749 (58.9) | 128 (57.1) | 212 (61.6) |
| Age > 40 | 1536 (29.4) | 347 (27.3) * | 68 (30.4) * | 112 (32.6) * |
| Employed | 1988 (38.0) | 396 (31.1) | 79 (35.3) * | 112 (32.6) * |
| Body mass index (kg/m2) | ||||
| < 18.5 | 815 (15.6) | 412 (32.4) | 68 (30.4) | 78 (22.7) |
| 18.5 - 24.9 | 2872 (55.0) | 707 (55.6) | 122 (54.5) | 201 (58.4) |
| 25.0 - 29.9 | 1049 (20.1) | 124 (9.8) | 25 (11.2) | 50 (14.5) |
| ≥ 30.0 | 490 (9.4) | 29 (2.3) | 9 (4.0) | 15 (4.4) |
| Low hemoglobin | 2280 (43.6) | 913 (71.8) | 168 (75.0) | 211 (61.3) |
| CD4 count (cells/mm3) | ||||
| Median (IQR) | 97 (37-166) |
59 (23-116) |
48 (13-112) |
64 (16-126) |
| Mean (95% CI) | 113 (110-116) |
80 (75-84) |
73 (62-83) |
81 (72-89) |
| < 50 | 1498 (28.7) | 525 (41.3) | 112 (50.0) | 139 (40.4) |
| 50 - 99 | 954 (18.3) | 303 (23.8) | 40 (17.9) | 72 (20.9) |
| 100 - 199 | 1729 (33.1) | 306 (24.1) | 49 (21.9) | 89 (25.9) |
| 200 - 349 | 489 (9.4) | 46 (3.6) | 10 (4.5) | 14 (4.1) |
| ≥ 350 | 112 (2.1) | 13 (1.0) | 3 (1.3) | 3 (0.9) |
| Missing | 444 (8.5) | 79 (6.2) | 10 (4.5) | 27 (7.9) |
| WHO stage IV | 412 (7.9) | 228 (17.9) | 49 (21.9) | 33 (9.6) * |
| Peripheral neuropathy | 138 (2.6) | 81 (6.4) | 11 (4.9) * | 11 (3.2) * |
Incident TB column comprises only subjects who had no TB at baseline. P-values are 2-sided by chi-square test compared to “No TB treatment”; means compared by t-test, medians by Wilcoxon rank sum test.
All comparisons were significant at p < 0.01 level except for those indicated with *.
After adjustment for altitude, lower limit of normal hemoglobin is 12.35/11.35/10.35 g/dl for men, women, and pregnant women respectively.
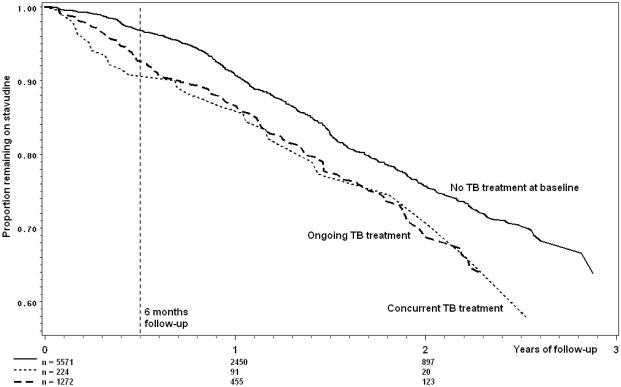
Among the 7,066 individuals, 260 individuals (3.7%) died, 1,252 (17.7%) became lost to follow-up, and 1,219 (17.3%) experienced stavudine substitutions. Of these, 842 were single-stavudine substitutions, 203 were multi-drug substitutions, and 172 were switches to second-line HAART (zidovudine/didanosine/lopinavir-ritanovir). The crude rate of single-stavudine substitution in the entire cohort was 12.4 (95% CI 11.6-13.3) per 100 person-years with a median time to single-stavudine substitution of 347 (IQR 175-535) days. As expected, absolute rate of stavudine substitution increased with time since HAART initiation, from months 0-6 (7.9, 95% CI 6.9-9.1 substitutions per 100 person-years), to months 6-12 (12.3, 95% CI 10.8-14.0), and to the rest of follow-up (18.1, 95% CI 16.4-20.0). This increasing rate is evident in Figure 2, which shows Kaplan-Meier curves for single-stavudine substitutions stratified by baseline TB treatment exposure category. These curves show an increased risk of stavudine substitution in the first months of concomitant TB and HAART compared to those not receiving TB treatment, and no increased risk due to TB treatment after 6 months (vertical dashed line); at the same time, these curves show that the absolute rate of stavudine substitution increases at six months and again at one year.
Figure 2.
Kaplan-Meier analysis of time to stavudine substitution by TB treatment status at time of HAART initiation, among 7,066 individuals initiating HAART in Johannesburg, South Africa.
Hazard ratios adjusted for confounding, loss to follow-up, death, and the competing risk of multi-drug substitution are shown in Table 2. Crude results were similar to adjusted results (data not shown). Among people with ongoing TB treatment at time of HAART initiation, adjusted hazard ratio (aHR) for stavudine substitution was 3.18 (95% CI 1.82-5.56) in the first two months of HAART, 2.51 (95% CI 1.77-3.54) for months 3-6, and 1.19 (95% CI 0.94-1.52) thereafter. Among people with concurrent initiation of treatment for TB and HAART, aHR was 6.60 (95% CI 3.03-14.37) in the first two months of HAART, 1.88 (95% CI 0.87-4.09) for months 3-6, and 1.07 (95% CI 0.65-1.76) thereafter. Among people with incident TB treatment after HAART initiation, the aHR was not different from the referent group and imprecise in all three time periods (Table 2). Early incident TB treatment tended to be associated with increased risk of stavudine substitution in the first two months of co-treatment with an aHR of 2.74 (95% CI 0.84-8.94).
Table 2.
Adjusted hazard ratios and 95% confidence intervals for the effect of TB treatment on stavudine substitution by timing of TB treatment initiation relative to HAART initiation and duration of co-treatment, among 7,066 individuals initiating HAART in Johannesburg, South Africa.
| TB treatment | n | Months of TB/HAART treatment | ||
|---|---|---|---|---|
| 0-2 | 3-6 | 7+ | ||
| No TB treatment | 1. | 1. | 1. | |
| Ongoing | ||||
| All | 1272 | 3.18 (1.82-5.56) |
2.51 (1.77-3.54) |
1.19 (0.94-1.52) |
| Long-term | 716 | 3.17 (1.58-6.34) |
2.13 (1.34-3.37) |
1.20 (0.89-1.62) |
| Short-term | 556 | 3.58 (1.74-7.35) |
3.17 (2.04-4.92) |
1.22 (0.84-1.78) |
| Concurrent | 224 | 6.60 (3.03-14.37) |
1.88 (0.87-4.09) |
1.07 (0.65-1.76) |
| Incident | ||||
| All | 349 | 0.99 (0.46-2.12) |
1.18 (0.61-2.25) |
0.87 (0.28-2.73) |
| Early | 132 | 2.74 (0.84-8.94) |
1.50 (0.53-4.27) |
1.58 (0.26-9.55) |
| Late | 217 | 0.59 (0.24-1.48) |
1.03 (0.45-2.39) |
0.67 (0.17-2.70) |
Almost half (362 or 43%) of the 842 single-stavudine substitutions were attributed to clinically-diagnosed peripheral neuropathy, 24% to lipodystrophy (n=205), 20% to lactic acidosis or symptomatic hyperlactatemia (n=168), 2% to a combination of these toxicities (n=21); the remainder (11%) had no reason recorded. Patients who switched due to peripheral neuropathy were more likely to have TB at HAART initiation or during follow-up (RR=1.53, 95% CI 1.33-1.75), whereas those who switched for lactic acidosis or lipodystrophy were less likely to have TB (RR=0.58, 95% CI 0.48-0.71).
Sensitivity analysis
Sensitivity analyses indicated that main results were not sensitive to absolute stavudine dose (30 or 40 milligrams), inclusion of multi-drug substitutions, or total TB treatment lasting nine months (rather than six). We found that restricting analysis to those without recorded history of TB resulted in slightly elevated hazard ratios for both ongoing and concurrent analyses in months 0-2 (ongoing: aHR=3.56 versus baseline 3.18; for concurrent, 7.57 versus 6.60). This suggested that lingering peripheral neuropathy from previous drug exposures might have biased the results toward the null.
DISCUSSION
Concomitant administration of TB treatment and HAART is common in sub-Saharan Africa, especially at time of initiation of HAART. Our results show that concurrent initiation of TB treatment and stavudine-based HAART within a 2 week window puts patients at a nearly seven-fold increased risk of substitution for stavudine in the first two months of HAART (aHR=6.60, 95% CI 3.03-14.37), and some increased risk until six months after HAART initiation. Likewise, initiation of stavudine in patients whose TB treatment was ongoing for more than 2 weeks at time of HAART initiation was associated with a two- to three-fold increased risk of stavudine substitution over the first six months of HAART. The presence of an effect of both ongoing and concurrent TB treatment within six months of HAART initiation are evident in crude results shown in Figure 2, where survival curves diverge sharply in the first six months, but are similar (essentially parallel) thereafter. In general, there was little to no effect of incident TB treatment on risk of stavudine substitution, although there was a suggestion that early incident TB might raise the risk of stavudine substitution. Risks remained elevated even among patients who received a twice daily 30mg dose of stavudine, per current WHO recommendations, and were not confounded by stavudine dose (milligrams per kilogram). These results, based on a large sample size and robust analytic methods, confirm and extend the results of two small studies from England, in which higher than expected rates of adverse events with stavudine and TB treatment were observed [27, 28].
Almost half (43%) of single-stavudine substitutions could be attributed to peripheral neuropathy; individuals receiving TB treatment were more likely than others to switch for reason of peripheral neuropathy (RR=1.53). This is consistent with the hypothesis that peripheral neuropathy is a key symptomatic pathway for the interaction of TB drugs and HAART. Peripheral neuropathy is caused by both isoniazid [10, 18] and stavudine [3, 14, 17, 18, 27, 28] through different mechanisms; use of both drugs may lead to an additive or cumulative effect, increasing severity of symptoms.
Indeed, these results may understate the true impact of stavudine and TB treatment, for three reasons: first, because the great majority of TB patients in South Africa are prescribed vitamin B6 (pyridoxine) at time of initiation of TB treatment for the prevention of peripheral neuropathy, and in addition, amitriptyline is frequently prescribed to manage incident peripheral neuropathy. It is possible that the effect of TB on the risk of stavudine substitution would be even higher in settings where these drugs were not routinely used; conversely rates of peripheral neuropathy may be further reduced with additional micronutrient supplementation [29].
Second, restricting the reference group to individuals without a history of TB yielded higher effect estimates for analysis of concurrent and ongoing TB treatment. This suggests that main analyses could have underestimated the true impact of TB treatment on risk of stavudine substitution, perhaps due to residual peripheral neuropathy among those with a recent history of TB treatment.
Third, while focusing on stavudine substitution as the outcome ensures that we capture the most severe toxicities, we did not examine the impact of TB treatment on risk of mild stavudine toxicity. It is likely that treatment for TB resulted in additional low level peripheral neuropathy, which may remain undiagnosed, unreported, or resolves without stavudine substitution. Thus, the impact of TB treatment on risk of stavudine toxicities may be higher than the effect of TB treatment on risk of stavudine substitution.
It was surprising that incident TB treatment, and in particular late incident TB treatment, was not associated with an increase in stavudine substitution; this may be the result of depletion of susceptible patients. An alternate hypothesis is that the effect of TB treatment on risk of stavudine-related toxicity is mediated by HIV viral load. Control of HIV replication would then reduce the effect of TB treatment on risk stavudine substitution.
TB treatment did not increase risk of stavudine substitution after six months (the duration of a typical course of TB treatment). However, the bulk of stavudine substitution occurs after six months; it is important to note that while the relative rate of stavudine substitution attributable to TB treatment was not significant after six months in any exposure group, the absolute rate of stavudine substitution increased substantially after both six and twelve months of HAART. A related point is that, because the absolute risk of stavudine substitution is relatively low in the six months following initiation of stavudine, even a large hazard ratio may translate into a relatively small absolute risk difference. Only 219 stavudine substitutions took place during the first six months following initiation of HAART among patients at risk for that period. The absolute risk in those with any TB treatment at baseline (ongoing or concurrent) was 5.7%, and in those without was 2.4% (risk difference 3.3%, 95% CI 2.0-4.5%), corresponding to one stavudine substitution for every 18 patients with TB treatment and one for every 42 patients without any TB treatment. Because of the large number of individuals who initiate HAART while on TB treatment (>20%) in this population, we believe these results remain of considerable public health relevance.
There were several limitations of this study. First, this observational study was conducted in a busy clinic setting using routinely collected data; results are therefore necessarily less definitive than they would be in a randomized trial. In particular, while we strove to control adequately for confounding, uncontrolled confounding may still be present, including exposure to alcohol and other drugs associated with peripheral neuropathy. Similarly, while we corrected for bias due to competing risks including death, LTFU, and multi-drug substitution, complete control of such bias depends on the difficult-to-verify assumption that competing risks can be completely explained by observed variables [24, 25]. Last, if patients receiving stavudine and isoniazid were evaluated more thoroughly for peripheral neuropathy than comparable patients, this might lead to detection or diagnostic bias that might result in an overestimation of the effect. Future studies of this topic should use blinded evaluation of peripheral neuropathy to eliminate this potential bias.
In conclusion, results show that in this patient population, concurrent initiation of TB treatment and HAART, as well as initiation of HAART while receiving ongoing TB treatment, is an important risk factor for stavudine substitution, irrespective of stavudine dose. These results suggest that screening for peripheral neuropathy is important in patients receiving d4T and TB treatment, especially those initiating both treatments within a short period. Moreover, in settings where alternative antiretroviral drugs are available, we may wish to reconsider the use of stavudine in first-line HAART among patients with ongoing or concurrent initiation of TB treatment.
Acknowledgements and funding
The clinical activities of the Helen Joseph Hospital are supported by the National and Gauteng Department of Health and the United States President's Emergency Plan for AIDS Relief (PEPFAR) in a grant by USAID to Right to Care and the Institution (674-A-00-08-00007-00). The research activities of this publication are supported by the National Institute for Health in a grant from the NIAID, DAIDS division (CIPRA IU19 AI53217-01, PEPFAR protocol #3 U19 AI 053217-04SI-R2C01). DW also received support from an unrestricted educational training grant from the UNC-GSK Center for Excellence in Pharmacoepidemiology and Public Health, UNC School of Public Health and NIH/NIAID 5 T32 AI 07001-31 Training in Sexually Transmitted Diseases and AIDS. These agencies had no involvement in the design, collection, analysis, or interpretation of data in this study or in writing this article or submitting it for publication.
We would like to thank all the staff at TLC for their unyielding efforts on behalf of the patients. We would like to thank all of our patients, for their continuing bravery in the face of overwhelming circumstances.
Appendix: details of statistical models
All adjusted hazard ratios were estimated using baseline-stratified stabilized inverse probability weighted marginal structural models, implemented as weighted pooled log-binomial models. This model was baseline-stratified (a non-standard term in the literature) in that we controlled for confounding by baseline factors by including these variables in the final structural (i.e., regression) model; thus, resulting effect estimates are not fully marginal. The approach is common in marginal structural models (e.g. [22]), and is expected to improve variance of the estimate as compared to a pure reweighting approach for these factors. In the analysis of incident TB treatment, we also controlled for confounding by time-varying factors using inverse probability of treatment weights, and used baseline factors to stabilize these weights.
Inverse probability weights were also used to control for non-confounding bias, including censoring (lost to follow-up, death, or switches to second-line HAART) and the competing risk of multi-drug substitution including stavudine. The inclusion of competing risk weights in overall inverse probability weights has only been described once previously in the literature [25], but the intuition behind such an application of inverse probability weights is straightforward, simply assuming multiple censoring categories.
Covariates considered for inclusion in all models included gender, ethnicity, employment status, age, history of antiretroviral therapy, history of TB; baseline measures of pregnancy, peripheral neuropathy, hemoglobin (adjusted for sex, pregnancy, and altitude), body mass index (BMI), CD4 cell count, and WHO stage (IV or other), calendar date, whether clinical care was free at time of treatment initiation (before/after October 2006), and stavudine dose (milligrams per kilogram). Time varying models (of the exposure of incident TB treatment, censoring, or competing risks) also considered time-updated measures of hemoglobin, CD4 count, BMI, and virologic success (measured only after month 6), and month of follow-up as both a continuous and categorical variable.
Possible confounding variables were removed from consideration using a backwards elimination approach with a combined criterion of log-scale change-in-estimate of 0.10 and a chi-square p-value >0.10. The final variables used to control confounding were (for both main exposures): gender, age, history of antiretroviral therapy, pregnancy, peripheral neuropathy at baseline, BMI, year of HAART initiation, and whether clinical care was free or not, and stavudine dose (milligrams per kilogram). We did not implement a selection process for models for stabilized censoring or competing risk weights; in both cases, all baseline variables and the exposure were included in stabilizing portions of weights (numerators), while all baseline and time-updated covariates as well as the exposure were included in weight models (denominators) [24]. All weights were estimated using logistic regression.
The positivity assumption requires that there be no combination of observed confounders (covariates) that predicts exposure (treated for TB, or not treated for TB) in a deterministic way [30]. We evaluated the positivity assumption by visually examining histograms of multivariate predicted probability of exposure by observed exposure status (a process equivalent to the comparison of propensity score distributions). Because there was substantial overlap in these curves, we judged that the positivity assumption was approximately met in these data for the exposures of interest.
Footnotes
Conflicts of interest.
DW: I received funding from an unrestricted educational training grant from the UNC-GSK Center for Excellence in Pharmacoepidemiology and Public Health, UNC School of Public Health. As noted above, GSK had no involvement in the design, collection, analysis, or interpretation of data in this study or in writing this article or submitting it for publication.
IS, MM, BMK, FC, PM, MJF, JSK, AVR, PM: no conflict.
REFERENCES
- 1.WHO. TOWARDS UNIVERSAL ACCESS . Scaling up priority HIV/AIDS interventions in the health sector. Geneva: 2007. Progress Report, April 2007. http://www.who.int/hiv/mediacentre/universal_access_progress_report_en.pdf. (Accessed 14 September 2007) [Google Scholar]
- 2.Bolhaar MG, Karstaedt AS. A high incidence of lactic acidosis and symptomatic hyperlactatemia in women receiving highly active antiretroviral therapy in Soweto, South Africa. Clin Infect Dis. 2007;45:254–60. doi: 10.1086/518976. [DOI] [PubMed] [Google Scholar]
- 3.Boulle A, Orrell C, Kaplan R, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12:753–60. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 4.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–42. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 5.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 6.Colebunders R, Kamya MR, Laurence J, et al. First-line antiretroviral therapy in Africa - how evidence-based are our recommendations? AIDS Rev. 2005;7:148–54. [PubMed] [Google Scholar]
- 7.Currier J. Management of metabolic complications of therapy. Aids. 2002;16(Suppl 4):S171–6. doi: 10.1097/00002030-200216004-00023. [DOI] [PubMed] [Google Scholar]
- 8.Currier JS. Sex differences in antiretroviral therapy toxicity: lactic acidosis, stavudine, and women. Clin Infect Dis. 2007;45:261–2. doi: 10.1086/518977. [DOI] [PubMed] [Google Scholar]
- 9.Currier JS, Havlir DV. Complications of HIV disease and antiretroviral therapy. Top HIV Med. 2005;13:16–23. [PubMed] [Google Scholar]
- 10.Subbaraman R, Chaguturu SK, Mayer KH, Flanigan TP, Kumarasamy N. Adverse effects of highly active antiretroviral therapy in developing countries. Clin Infect Dis. 2007;45:1093–101. doi: 10.1086/521150. [DOI] [PubMed] [Google Scholar]
- 11.Falco V, Rodriguez D, Ribera E, et al. Severe nucleoside-associated lactic acidosis in human immunodeficiency virus-infected patients: report of 12 cases and review of the literature. Clin Infect Dis. 2002;34:838–46. doi: 10.1086/339041. [DOI] [PubMed] [Google Scholar]
- 12.Parienti JJ, Massari V, Descamps D, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38:1311–6. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 13.Forna F, Liechty CA, Solberg P, et al. Clinical toxicity of highly active antiretroviral therapy in a home-based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr. 2007;44:456–62. doi: 10.1097/QAI.0b013e318033ffa1. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins C, Achenbach C, Fryda W, Ngare D, Murphy R. Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. J Acquir Immune Defic Syndr. 2007;45:304–10. doi: 10.1097/QAI.0b013e318050d66c. [DOI] [PubMed] [Google Scholar]
- 15.WHO . TB/HIV: A Clinical Manual. Second edition. Geneva: 2004. (Accessed March 31 2008) [Google Scholar]
- 16.South African National Department of Health National Antiretroviral Treatment Guidelines. HIV and AIDS Policy Guideline. (1st Edition) 2004 [Google Scholar]
- 17.Amoroso A, Sheneberger R, Fielder AEJ, Etienne M, Stafford K. ART-Associated Toxicities Leading to a Switch in Medication: Experience in Uganda, Kenya, and Zambia; 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. Abstract 789. [Google Scholar]
- 18.Moyle GJ, Sadler M. Peripheral neuropathy with nucleoside antiretrovirals: risk factors, incidence and management. Drug Saf. 1998;19:481–94. doi: 10.2165/00002018-199819060-00005. [DOI] [PubMed] [Google Scholar]
- 19.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007:CD005454. doi: 10.1002/14651858.CD005454.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO . Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a public health approach. Geneva: 2006. 2006 revision. http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. (Accessed 14 October 2007.) [PubMed] [Google Scholar]
- 21.Cole SR, Hernán MA, Anastos K, Jamieson BD, Robins JM. Determining the effect of highly active antiretroviral therapy on changes in human immunodeficiency virus type 1 RNA viral load using a marginal structural left-censored mean model. Am J Epidemiol. 2007;166:219–27. doi: 10.1093/aje/kwm047. [DOI] [PubMed] [Google Scholar]
- 22.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Matsuyama Y, Yamaguchi T. Estimation of the marginal survival time in the presence of dependent competing risks using inverse probability of censoring weighted (IPCW) methods. Pharm Stat. 2007 doi: 10.1002/pst.290. [DOI] [PubMed] [Google Scholar]
- 26.Republic of South Africa Department of Health National Antiretroviral Treatment Guidelines. HIV and AIDS Policy Guideline. 2004 [Google Scholar]
- 27.Breen RA, Lipman MC, Johnson MA. Increased incidence of peripheral neuropathy with co-administration of stavudine and isoniazid in HIV-infected individuals. Aids. 2000;14:615. doi: 10.1097/00002030-200003310-00017. [DOI] [PubMed] [Google Scholar]
- 28.Dean GL, Edwards SG, Ives NJ, et al. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. Aids. 2002;16:75–83. doi: 10.1097/00002030-200201040-00010. [DOI] [PubMed] [Google Scholar]
- 29.Villamor E, Mugusi F, Urassa W, et al. A Trial of the Effect of Micronutrient Supplementation on Treatment Outcome, T Cell Counts, Morbidity, and Mortality in Adults with Pulmonary Tuberculosis. J Infect Dis. 2008;197:1499–1505. doi: 10.1086/587846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernan MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60:578–86. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]