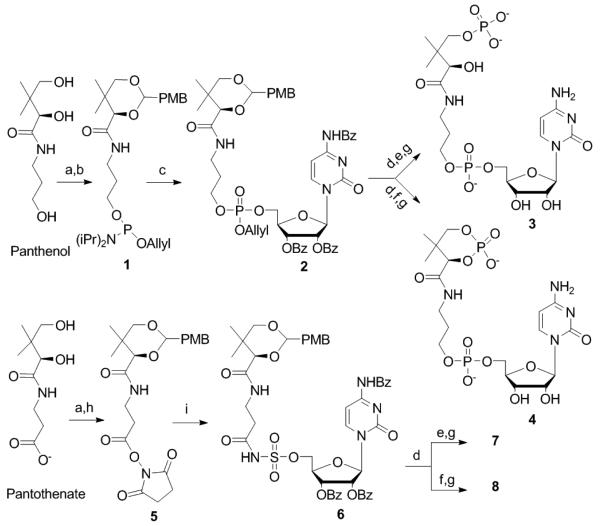
Figure 2.
Synthesis of inhibitors: a) (MeO)2CHC6H4OMe, CSA, DMF b) Allyl-O-P[N(i-Pr2)]2, 5-(Ethylthio)-1H-tetrazole, DCM c) 1) 5-(Ethylthio)-1H-tetrazole, HO-2′,3′,N4-tribenzoyl cytidine, CH3CN 2) CSO, 0°C d) 80% AcOH e) 1) pyridinium HCl, β-cyanoethyl-O-P[N(iPr2)]2, CH3CN, −20°C 2) CSO, 0°C f) 1) 5-(Ethylthio)-1H-tetrazole, β-cyanoethyl-O-P[N(i-Pr2)]2, CH3CN 2) CSO, 0°C g) 1) TMSCl, DBU, CH3CN 2) NH4OH, 55°C h) NHS, DCC, THF i) Cs2CO3, NH2SO2-2′,3′,N4-tribenzoyl cytidine, DMF.