Figure 10.
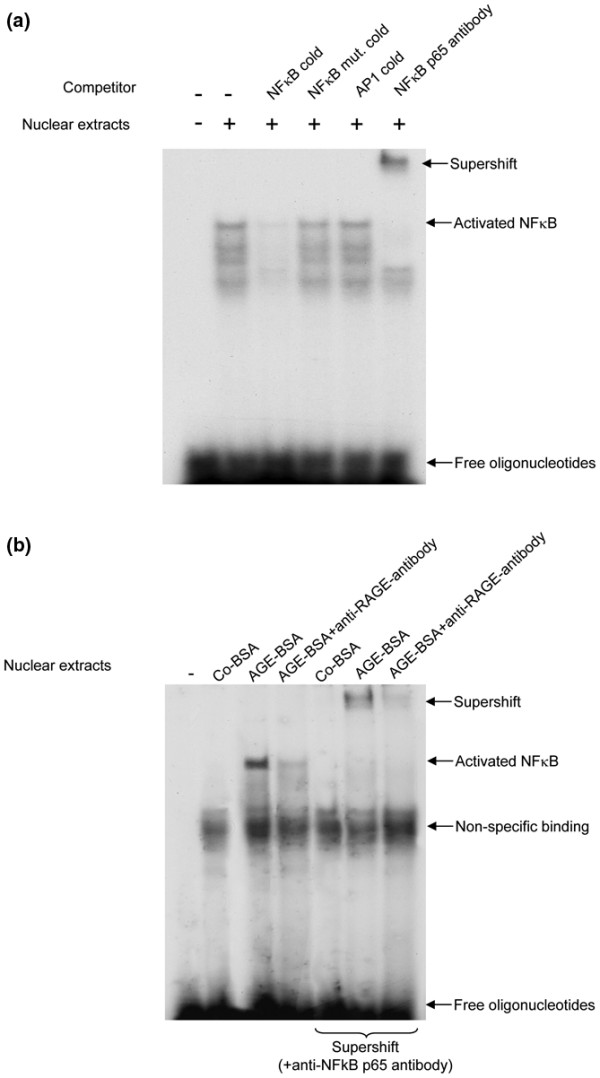
EMSA for NFκB. (a) A control experiment was performed to demonstrate the specificity of the assay. Aliquots of the nuclear extracts of TNF-α-activated fibroblast-like synovial cells (FLS; 10 ng/ml TNF-α for two hours) were incubated without (-) or with the indicated unlabelled oligonucleotides in the competition assays. The DNA-binding was reduced in the presence of cold nuclear factor kappa B (NFκB) probe, but not with NFκB mutant or AP1 oligonucleotides. An anti-NFκB p65 antibody induced a supershift. Data are representative of three separate experiments. (b) Advanced glycation end products-modified (AGE)-BSA, but not control-BSA (Co-BSA) treatment results in NFκB activation and the formation of NFκB-DNA complexes. Nuclear proteins isolated from AGE-BSA treated FLS in the presence of a receptor for AGEs (RAGE)-neutralising antibody showed a weaker NFκB binding in comparison with AGE-BSA stimulation alone. This demonstrates that the AGE-induced NFκB activation was caused by AGE-RAGE interactions. The specificity of NFκB binding was confirmed by supershifts using the NFκB p65 antibody. The shown electrophoretic mobility shift assay (EMSA) is representative of two independent experiments with similar results.
