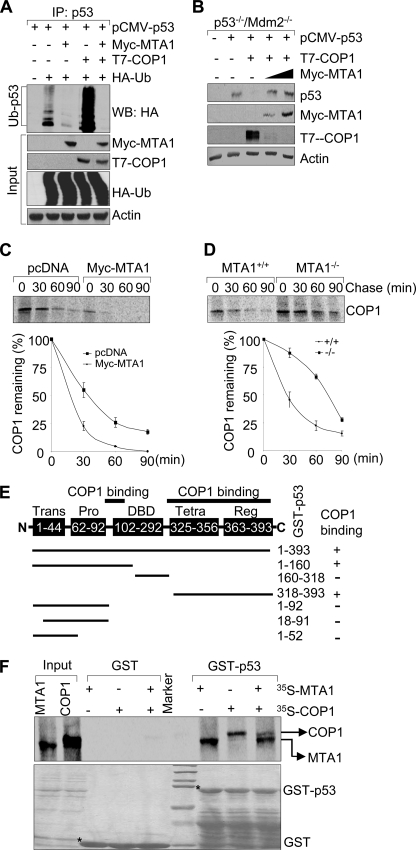
FIGURE 4.
MTA1 inhibits the COP1-mediated p53 ubiquitination and degradation. A, HEK293 cells were transfected with the indicated plasmids. After 36 h of transfection, total cell lysates were prepared and subjected to the sequential IP/Western blot analysis with the indicated antibodies. B, p53−/−/Mdm2−/− double-null MEFs were transfected with the indicated expression vectors and immunoblotted with the indicated antibodies. C and D, U2OS cells transfected the indicated expression vectors (C) or MTA1+/+ and MTA1−/− MEFs (D) were labeled using [35S]methionine and subjected to pulse chase analysis as described above except that immunoprecipitations were carried out using an anti-COP1 antibody. E, schematic representation of the domains of p53 protein for COP1 binding. Trans, transactivation domain; Pro, proline-rich domain; DBD, DNA binding domain; Tetra, tetramerization domain; Reg, regulatory domain. F, in vitro competition binding assay of MTA1 and COP1 to p53. In vitro-translated 35S-labeled MTA1 or COP1 protein was incubated with GST or GST-p53-fused protein. Bound proteins were separated and analyzed by SDS-PAGE and autoradiography.