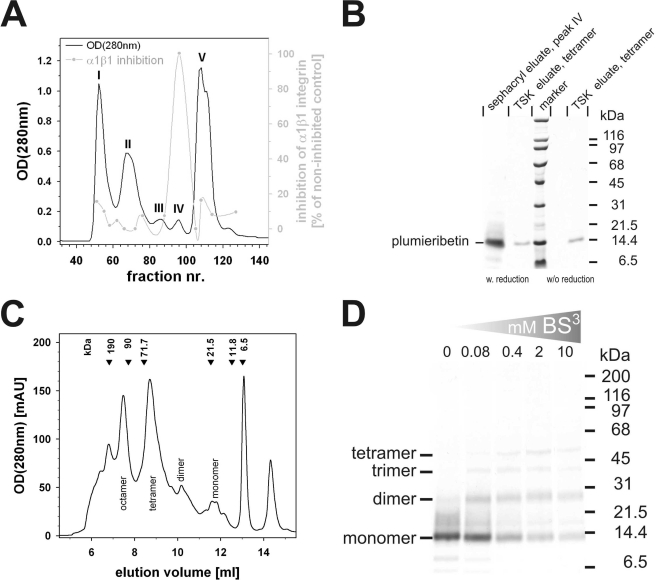
FIGURE 2.
Purification of the α1β1 integrin-inhibiting factor from S. plumieri venom and studies of its quaternary structure. The crude venom was separated by gel filtration on a Sephacryl S-200 HR column into five fractions, labeled I–V (A). The α1β1 integrin-inhibiting factor was eluted in fraction IV, which runs as a 14-kDa band in SDS-PAGE (B). analytical gel filtration on a calibrated TSK S2000 size exclusion column provided several peaks (C), most of which contain the same 14-kDa protein (shown by SDS-PAGE in (B)), yet in different oligomeric states (monomer, dimer, tetramer, and octamer). Calibration marker proteins with their molecular masses are indicated at the elution volumes above the chromatogram. Cross-linkage studies with the homobifunctional cross-linker BS3 proved the oligomeric nature of the α1β1 integrin-inhibiting factor in solution (D).