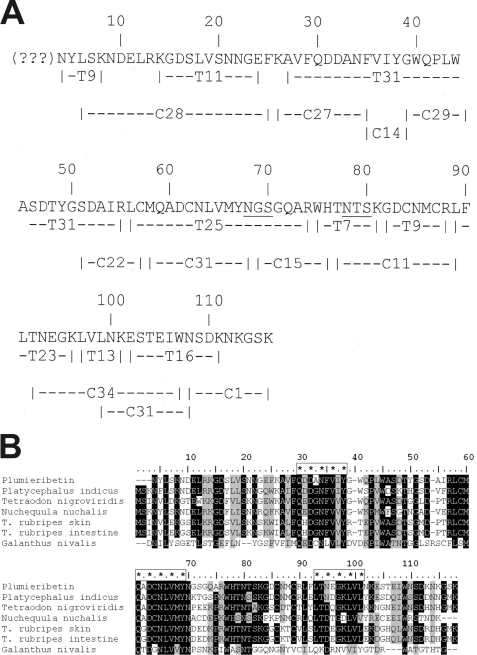
FIGURE 4.
Primary structure of plumieribetin (A) and its alignment with homologous fish skin proteins and the prototypic monocot B-type lectin from snow drop (B). Tryptic and chymotryptic fragments (indexed by initial letters T and C, respectively) of plumieribetin were sequenced by Edman degradation and aligned to obtain almost the full sequence of the fish venom component. The N-terminal three amino acids could not be sequenced by Edman degradation and are indicated by question marks (A). Found by BLAST search, homologous fish proteins from P. indicus, Tetradon nigroviridis, Nuchequula nuchalis, a skin protein and an intestinal protein from T. rubripes, as well as from the monocot plant snow drop (G. nivalis) showed remarkable homology to plumieribetin (B). The sequences were aligned by the software BioEdit using the ClustalW algorithm. Similar sequence (threshold above 75%) and identical residues are shaded gray and black, respectively. The three putative mannose-binding sites are marked with enframed asterisks.