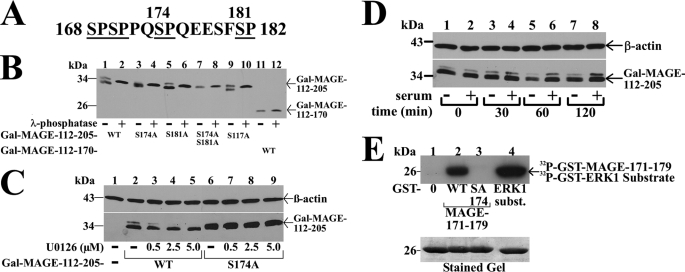
FIGURE 5.
Serum stimulation of MAP kinase phosphorylation of MAGE-11 Ser-174. A, MAGE-11 Ser-Pro sites Ser-168, Ser-170, Ser-174, and Ser-181 (underlined) include consensus MAP kinase site 172PQSP175. B, GAL-MAGE-(112–205) wild-type (WT) and mutants and GAL-MAGE-(112–170) (5 μg) were expressed in COS cells. Cell extracts in IP lysis buffer without sodium fluoride (35 μg) were incubated in the absence and presence of 800 IU of λ-phosphatase for 1 h at 4 °C, and the immunoblot probed with GAL antibody. C, GAL0 (—), GAL-MAGE-(112–205) WT, or S174A mutant (5 μg) were expressed in COS cells. Immediately after transfection and the next day, serum-free medium containing 1 μm MG132 and U0126 was added. Cells were extracted in IB lysis buffer containing phosphatase mixture inhibitors 1/2. Protein extracts (35 μg of protein/lane) were analyzed on immunoblots probed using GAL and β-actin antibodies. D, GAL-MAGE-(112–205) (5 μg) was expressed in COS cells incubated in the absence (lanes 1, 3, 5, and 7) or presence of 10% fetal bovine serum (lanes 2, 4, 6, and 8). Cells were harvested at 0, 30, 60, and 120 min in IB lysis buffer. Cell extracts (35 μg of protein/lane) were analyzed by immunoblots probed with GAL and β-actin antibodies. E, ERK1 in vitro phosphorylation of MAGE-11 Ser-174. In vitro kinase assay (top panel) was performed as described (19) at 30 °C for 30 min with 0.16 μCi/μl of [γ-32P]ATP, 0.1 μg of active ERK1 kinase and purified GST0 (lane 1), GST-MAGE-(171–179) WT (lane 2), and S174A mutant (lane 3), and ERK1 substrate modeled after myelin basic protein (37) (lane 4). The dried gel was exposed to x-ray film for 16 h, and rehydrated for Coomassie Blue staining to demonstrate equivalent protein loading (bottom panel).