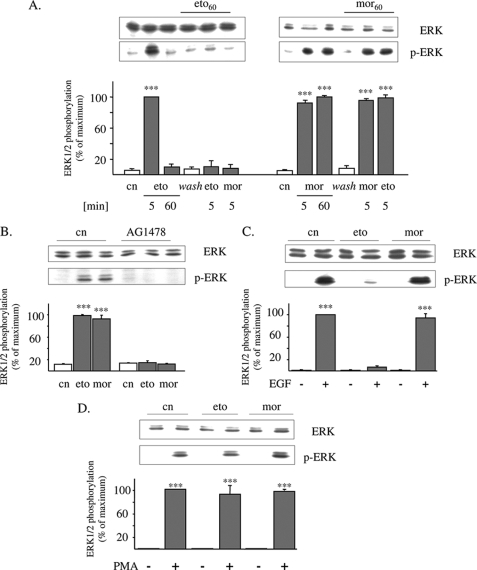
FIGURE 1.
Etorphine, but not morphine, desensitizes EGF receptor activation of ERK1/2. A, serum-starved HEK/DOR cells were exposed to 1 μm morphine or 100 nm etorphine for 5 and 60 min to stimulate ERK1/2 signaling. In some experiments, the cells were first pretreated with morphine (1 μm; mor60) or etorphine (100 nm; eto60) for 60 min, washed, and subsequently stimulated with the opioids indicated for 5 min. Controls (cn) were left untreated. The cells were lysed by the addition of sample buffer and examined for ERK1/2 activation by Western blot using of a phospho-specific antibody (p-ERK). B, HEK/DOR cells were pretreated with the EGF receptor inhibitor AG1478 (5 μm; 15 min) before 100 nm etorphine (eto) and 1 μm morphine (mor) were added for 5 min to determine ERK1/2 phosphorylation. Controls (cn) were left untreated. The cell lysates were prepared and analyzed for total (ERK) and phosphorylated ERK1/2 (p-ERK). C, serum-starved HEK/DOR cells were pretreated with etorphine (100 nm; eto) and morphine (1 μm; mor) for 60 min, washed, and subsequently stimulated with EGF (10 ng/ml) for 5 min. Controls (cn) received EGF alone. D, serum-starved HEK/DOR cells were incubated with 100 nm etorphine and 1 μm morphine for 60 min before ERK1/2 activation by cell exposure to 100 nm phorbol 12-myristate 13-acetate (PMA) for 5 min was evaluated. The cells of the same passage treated with the vehicle alone served as controls (cn). Stimulation of ERK1/2 activity was measured by Western blot using a phospho-specific antibody. Equal protein load was verified by analyzing the samples with an overall reactive ERK1/2 antibody. In each experiment, the intensity of phospho-ERK1/2 immunoreactivity was scanned and quantitated by video densitometry. ERK1/2 activation is expressed as the percentage of change from maximum stimulation, which was set to 100%. The data shown are the mean values ± S.D. from at least three independent experiments. ***, p < 0.001 versus nontreated controls (cn).