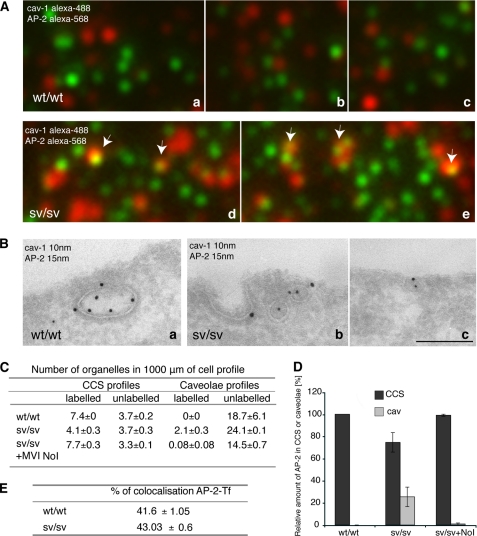
FIGURE 10.
The clathrin adaptor protein, AP-2, relocalizes into caveolae when myosin VI is lost. A, wt/wt and sv/sv fibroblasts were fixed under steady state condition and labeled with anti-caveolin-1 anti-AP-2 antibodies. The samples were observed by TIRF microscopy. The figure shows different fields of the wt/wt (a–c) and sv/sv (d and e) labeled samples. In sv/sv images, AP-2 co-localizes with caveolin-1 (arrows), whereas in the wt/wt images they are completely independent. B, similar experiment was performed by immuno-EM. Cryosections of wt/wt (a) and sv/sv (b and c) fibroblasts were double labeled with anti-caveolin-1 (cav-1) and anti-AP-2 antibodies. Using this different technique a relocalization of the clathrin adaptor protein AP-2 in caveolae was also observed. Bars: a, 300 nm; b, 200 nm; c, 300 nm. C, to quantify this observation a morphometric analysis was carried out on AP-2 labeling in CCS or caveolae organelles in 1000 μm of cell profiles of wt/wt and sv/sv fibroblasts. AP-2 localization on clathrin-coated structures was restored when the myosin VI NoI isoform was re-expressed in sv/sv fibroblasts. D, the relative amounts of AP-2 in CCS or caveolae in two independent experiments ± S.D. are shown. E, wt/wt and sv/sv fibroblasts were loaded with Tf-Alexa-555 and labeled with anti-AP-2 antibody. 500 fields of each sample were scanned by TIRF microscope and analyzed by Volocity software. The percentage of co-localization between AP-2 and transferrin does not change between wt/wt and sv/sv fibroblasts, revealing that the clathrin adaptor follows the transferrin receptor whether it is internalized by clathrin or caveolae.