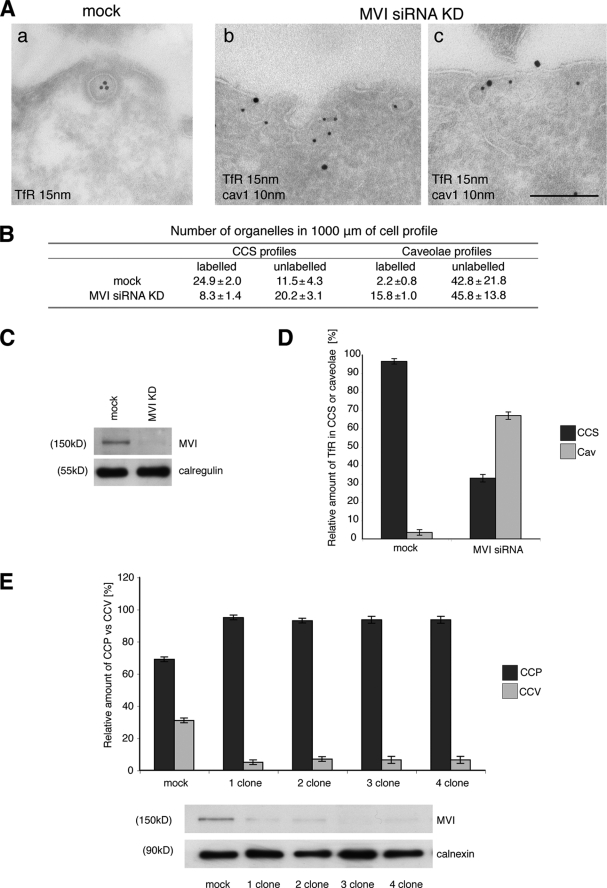
FIGURE 7.
Depletion of myosin VI in HeLa cells using siRNA knockdown relocates the TfR from CCS to caveolae. HeLa cells were either mock-transfected or transfected twice with siRNAs specific to myosin VI or to myosin VI and caveolin-1. A, mock-treated HeLa cells and KD cells were loaded with mouse anti-TfR antibody at 37 °C before fixation and processing for immuno-EM. Ultrathin cryosections were double labeled with anti-caveolin-1 and rabbit anti-mouse antibodies to visualize TfR. Representative electron micrographs show the localization of the TfR in CCS in control cells and co-localization with caveolin-1 in myosin VI KD cells. Bars: a, 250 nm; b, 200 nm; c, 200 nm. B, to quantify this observation a morphometric analysis was carried out to determine the numbers of TfR-labeled and unlabeled organelles in 1000 μm of cell profiles of mock-treated and myosin VI KD cells. The relative amounts of TfR in CCS or caveolae in two independent experiments ± S.D. are shown. C, the amount of myosin VI in the cells is shown in the Western blot with calregulin as a loading control. D, the histogram shows the relative amounts of TfR in caveolae and CCS in mock and siRNA MVI KD cells. E, HeLa cells were transfected with SureSilencingTM myosin VI small hairpin RNA plasmids (SuperArray-Bioscience Corp.) carrying the puromycin resistance gene. The puromycin resistance HeLa cell population was subcloned, and four clones expressing no significant level of myosin VI were selected. The four clones and the negative control were fixed under steady state conditions using the same labeling protocol with ruthenium red/glutaraldehyde described previously for mouse fibroblasts. All four HeLa clones that down-regulate myosin VI show a 5–6-fold reduction in clathrin-coated vesicle (CCV) internalization compared with the negative control. CCP, clathrin-coated pits.