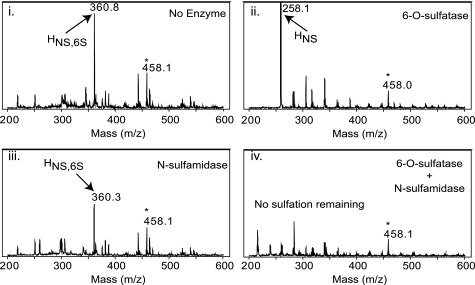
FIGURE 2.
Obligatory substrate-product relationship of N-sulfamidase. Desulfation of the disulfated monosaccharide HNS,6S (GlcNS,6S) by the enzymes was followed by electrospray mass spectrometry. Panel i, substrate only shown here as the sodium adduct of a single ion species (M − 1) with molecular mass of 360.8 Da; panel ii, treatment of GlcNS,6S substrate with 6-O-sulfatase desulfates the 6-O position; panel iii, inability of N-sulfamidase to hydrolyze the original disulfated monosaccharide (compare with panel i); panel iv, co-treatment of the disulfated substrate with both 6-O-sulfatase and N-sulfamidase enzymes showing the disappearance of all sulfated monosaccharides and demonstrating a prerequisite 6-O-desulfation by the 6-O-sulfatase prior to sulfate hydrolysis at the 2-amino position by the N-sulfamidase. Internal standard (458.1 Da) used to monitor ionization efficiency and mass calibration is noted by an asterisk.